Received: April 2021
DOI 10.17677/fn20714807.2021.02.03
Fluorine Notes, 2021, 135, 5-6
SYNTHESIS AND STUDY OF ELECTROCHEMICAL PROPERTIES OF 2-CHLORO-3-POLYFLUOROALCOXY- AND 2,3-BIS (POLYFLUOROALCOXY)-[1,4]-NAPHTHOQUINONES
S. M. Peregudova, V. I. Dyachenko
A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Vavilov St. 28, 119991, GSP-1, Moscow, Russian Federation
e-mail: vic-d.60@mail.ru
Abstract: The electroreduction of newly synthesized 2-polyfluoroalkoxy-3-chloro [1,4-naphthoquinones 1-3, 7 and 2,3-bis (polyfluoroalkoxy -[1,4]-naphthoquinones 4-6 was studied by cyclic voltammetry in aprotic medium (DMF). It was found that the reduction potential of E01 [1,4]-naphthoquinones 1-3, 7 does not depend on the length of polyfluoroalkyl substituent and is -0.35 V, while for naphthoquinones 4-6 it is 0.43 V, respectively. It was also found that the reduction potential of non-fluorinated [1,4]-naphthoquinones 8, 9 is greater than that of fluorinated analogs 1-7.
Keywords: reduction potential, cyclic voltammetry, 2-polyfluoroalkoxy-3-chloro-[1,4]-naphthoquinones, 2,3-bis (polyfluoroalkoxy)-[1,4]-naphthoquinones
Introduction
[1,4]-Benzo- and naphthoquinones are widespread in nature substances that play an important role in redox processes, cellular respiration and energy metabolism. Many of these substances are antioxidants, plant growth stimulants, antibiotics and anticancer drugs that have found its application in medicine [1, 2]. Their most important property is the ability to neutralize radicals formed when lipids are exposed to reactive oxygen species [3]. The most famous of them in the flora and fauna are phylloquinone and tocopherol (see Fig. 1) [4]. Lack of vitamin E leads to diseases of the nervous system, muscle weakness, infertility, cancer and cardiovascular diseases.
Figure 1. Antioxidants phylloquinone and tocopherol.
The quinoid system of these compounds plays a key role in providing energy to a living cell. Energy metabolism usually occurs on the surface and inside the mitochondrial membrane. Therefore, the ability of antioxidants to penetrate the mitochondrial membrane is very important for maintaining the cell electrochemical balance of a living organism. This largely depends on the charge and lipophilicity of polymethylene quinone substituent [5].
The most important reaction of naphthoquinones is its ability to reversibly reduce to dihydric phenols or naphthols. Their restoration is carried out in two stages. At the first stage, as a result of one-electron transfer, the radical anions and semiquinones are formed [6]. At the second stage, the radical anion adds another electron to form a dianion, which in a protic medium is converted to hydroquinone or 1,4-dihydroxynaphthalene (see Fig. 2).
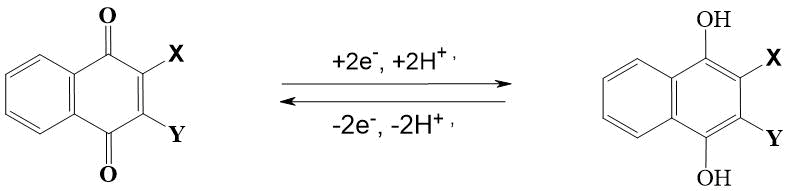
Figure 2. Conversion of quinones to hydroquinones.
The ability of quinoid system to reduce significantly depends on the donor-acceptor properties of the X, Y-substituents. The reduction energy (E0) is estimated using normal redox potential determined from the Nernst equation [7].
Thus, E0 is a quantitative characteristic of oxidizing ability of quinone, which is important for assessing the prospects for its use.
Results and discussion
In this article, the authors the features of the reduction of new fluorine-containing 1,4-naphthoquinones 1-7. Table 1 shows the values of normal redox potentials Eo for some recently [8] and newly synthesized 2-chloro-3-(polyfluoro) alkoxy and bis(polyfluoroalkoxy)-[1,4]-naphthoquinones, as well as their non-fluorinated analogs.
All studied compounds 1-11 are reduced with sequential reversible addition of two electrons. The result obtained is in complete agreement with studies carried out earlier for other naphthoquinones [9]. Indeed, in the first stage of reduction, the corresponding semiquinone is formed, which is further reduced to the corresponding dianion. The reduction potentials -E11/2 and -E21/2 of studied compounds are presented in Table 1. These data show that the reduction potentials of 1,4-naphthoquinone 10 are significantly shifted to the negative potential region compared to the reduction potentials of 2,3-dichloronaphthoquinone 11. The presence of two chlorine atoms in 1,4-naphthoquinone molecule ("control" compound) facilitates the reduction process by 60 mV. The substitution of one chlorine atom in compound 11 by Rf-CH2-O-group, where Rf = CF3 (1), CF2CF2H (2) and (CF2)5CF3 (3), leads to a shift of reduction potentials to negative region by 100 mV. Obviously, that substituents O-CH2-Rf, to a greater extent than Сl, exhibit the electron-donating properties and significantly complicate the reduction process.
As it turned out, the length of fluoroalkyl substituent does not significantly affect the values of reduction potentials for [1,4]-naphthoquinones 1-3, which indicates the absence of intramolecular charge transfer in compounds of this structure type.
For clarity, Fig. 3 shows the cyclic volamperograms of 1,4-naphthoquinones 1, 4, 11 obtained in DMF. Compounds 2, 3, 5-10 have the cathodic curves similar in shape.
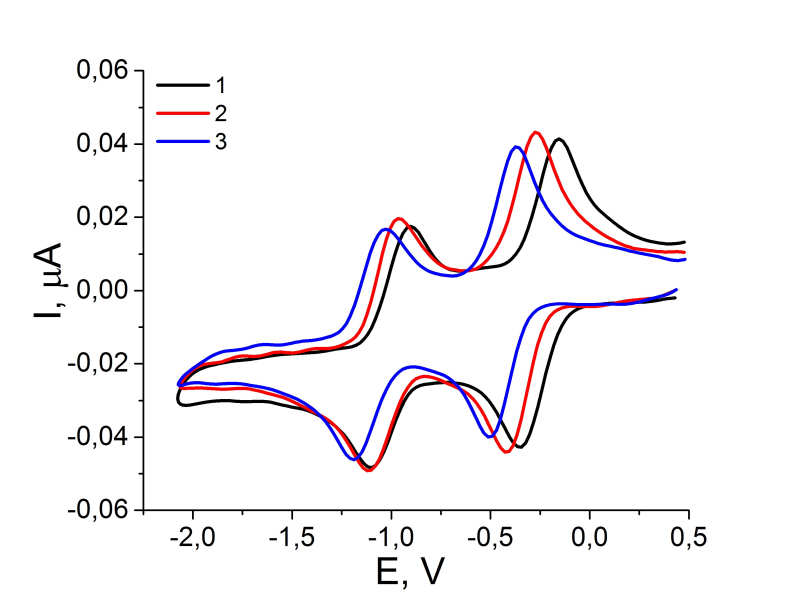
Figure 3. Cyclic voltammograms of reduction of 2,3-dichloro-[1,4]-naphthoquinone (1), 2-chloro-3-(2,2,2-trifluoroethoxy)-[1,4]-naphthoquinone (2) and 2,3-bis-(2,2,2-trifluoroethoxy)-[1,4]-naphthoquinone (3)
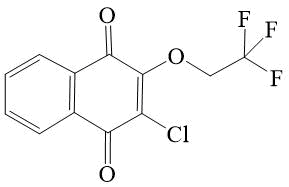
To verify the correctness of the judgment about the absence of influence of [1,4]-naphthoquinone substituent size on -E11/2 and –E21/2, we synthesized a new 2-chloro-3-(2,2,3,3,4 , 4,5,5,6,6,7,7,8,8,8,9,9-hexadecafluorononyloxy)-[1,4]naphthoquinone 3а (see Table 1), which differs from compounds 1-3 in noticeable length of fluorine-containing substituent (see Scheme 1). Its yield was over 80%. Studies undertaken have shown that there is indeed no significant difference in reduction potentials of naphthoquinones 1 and 7 (see Table 1).
Table 1. Reduction potentials of 2,3-substituted-[1,4]-naphthoquinones 1-11.
|
No |
Compound |
Reduction potential |
||
|
- E11/2, V |
- E21/2, V |
|||
|
1 |
2-chloro-3-(2,2,2-trifluoroethoxy)-[1,4]-naphthoquinone 1
|
0.35 |
1.04 |
|
|
2 |
2-chloro-3-(2,2,3,3-tetrafluoroprop-oxy)-[1,4]-naphthoquinone 2 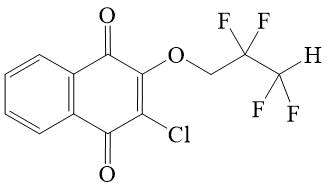
|
0.34 |
1.04 |
|
|
3 |
2-chloro-3-(2,2,3,3,4,4,5,5,6,6,7,7-tridecafluoroheptyloxy)-[1,4]-naphthoquinone 3 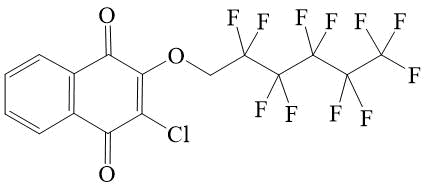
|
0.35 |
1.05 |
|
|
4 |
2,3-bis-(2,2,2-trifluoroethoxy)-[1,4]-naphthoquinone 4 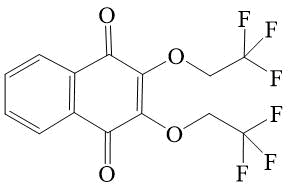
|
0.44 |
1.09 |
|
|
5 |
2,3-bis-(2,2,3,3-tetrafluoroprop-oxy)-[1,4]-naphthoquinone 5 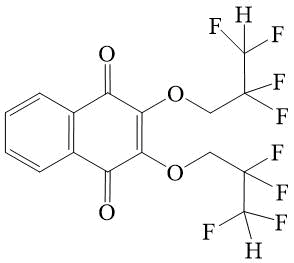
|
0.43 |
1.09 |
|
|
6 |
2,3-bis-(2,2,3,3,4,4,5,5-octafluoro-pentyloxy)-[1,4]-naphthoquinone 6 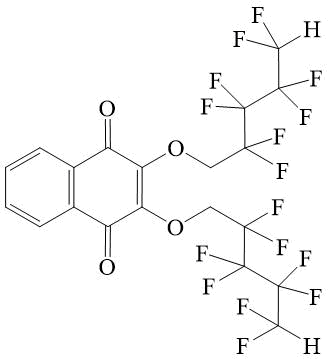
|
0.43 |
1.09 |
|
|
7 |
2-chloro-3-(2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9-hexadecafluorononyloxy)-[1,4]-naphthoquinone 7 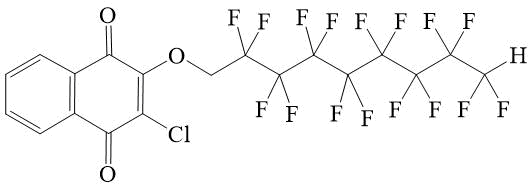
|
0.35 |
1.03 |
|
|
8 |
2-chloro-3-ethoxy-[1,4]-naphthoquinone 8 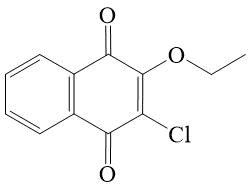
|
0.39 |
1.05 |
|
|
9 |
2-butoxy-3-chloro-[1,4]-naphthoquinone 9 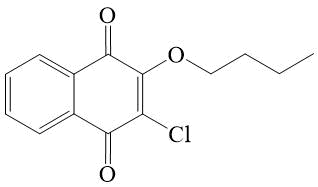
|
0,40 |
1,09 |
|
|
10 |
1,4-naphthoquinone* 10 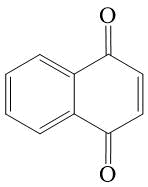
|
0.31 |
0.96 |
|
|
11 |
Dichloronaphthoquinone ("control" compound) 11 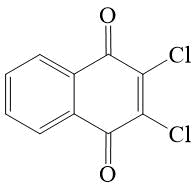
|
0.25 |
1.01 |
|
*Fritisch J., Tatwawadi S., Adams R., J.Phys. Chem., 1967, 71, 338.
Substitution of second chlorine atom by O-CH2-Rf substituent leads to further complication of reduction processes by 90 mV. In this case, the potentials of compounds 4-6 are close to each other, which confirm the absence of electronic effects transfer of alkyl substituents to reduction center. Moreover, the cyclic voltammograms of [1,4]-naphthoquinones 2, 3, 5-10 and 1, 4, 11 are similar in shape (see Fig. 2) and differ only in physical characteristics (see Table 1).
To describe the physicochemical properties of the synthesized compounds 1-3, 7 it is essential to compare the redox properties of polyfluorinated and non-fluorinated [1,4]-naphthoquinones.
In this regard, we have synthesized derivatives of [1,4]-naphthoquinones 3b and 3c, similar in structure to described earlier and not containing fluorine atoms in the molecule (see Scheme 1).
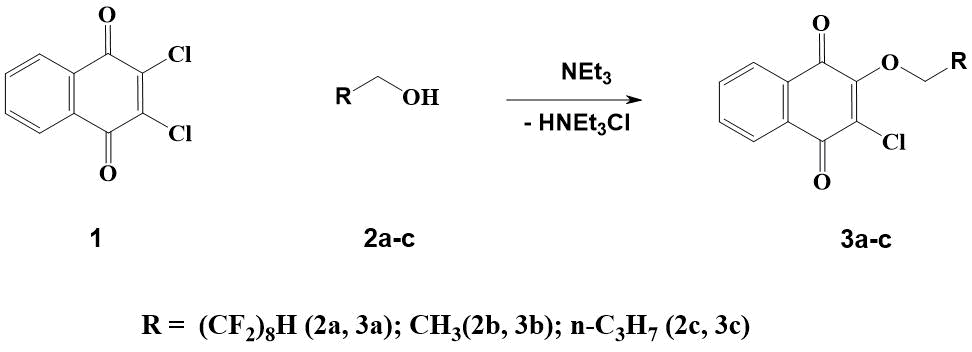
Scheme 1. Synthesis of [1,4]-naphthoquinones 3a-c.
According to the methods available in literature for synthesis of 3-chloro-2-ethoxy-[1,4]-naphthoquinone 3b, its yield does not exceed 15% [10, 11].
The authors found, that after heating 1 in ethanol at a temperature of 60-70°C in the presence of NEt3 for 1.5 hours, 3b is formed with a yield of more than 90%.
Depending on the method of obtaining 2-butoxy-3-chloro-[1,4]-naphthoquinone (3c), in one case it is stated as a liquid [12], and in the other - as a semi-solid substance [13].
In this regard, we synthesized compound 3с by heating 2,3-dichloro-[1,4]-naphthoquinone in butanol at 65-70°С in the presence of NEt3 according to a similar procedure for preparation of 3b. In this case, 3 was isolated in 65% yield as a solid substance (under normal conditions).
The naphthoquinones 3b,c, synthesized in this way, were studied by cyclic voltammetry analogously to compounds 8,9, respectively.
Moreover, for a comparative assessment of effect on quinoid system of substituents - OCH2-Rf and -OCH2-Alk, the reduction of 8 and 9 was carried out under the same conditions. It turned out that the recovery potentials of 8 and 9 are close to each other (see Table 1). At the same time, in comparison with the reduction potentials of compounds 1-3, 7 with polyfluoroalkoxy substituents, the potentials of 8 and 9 are shifted by 50 mV to the negative region. Obviously, this can be explained by the somewhat large electron-donor effect of OAlk groups not containing fluorine atoms.
The identity of reduction potentials in in vitro experiments (in series of naphthoquinones 1-3, 7, as well as in series 4-6), does not at all mean their identical activity in the body. In a living cell, the hydrophilicity-hydrophobicity of cytoplasm, the structure of radical (that must be neutralized), the size and chemical nature of antioxidant substituents, etc. are of great importance. In other words, in this case we are talking about its bioavailability. Indeed, in vitro antioxidant activity of tocotrienols (TCT) is higher than that of alpha-tocopherol, however, in a living organism, the activity of alpha-tocopherol is many times higher than that of TCT and other antioxidants of this type [14, 15].
The synthesized polyfluoroalkoxy-substituted [1,4]-naphthoquinones 1-7 can also be used as antioxidant additives in polymers and composites based on them. In particular, the antioxidant additives can be incorporated into fluoropolymers, in which traditional non-fluorinated antioxidants cannot be dissolved at the proper concentration.
Conclusions
As a result of electrochemical studies, it was found that 3-chloro-2-polyfluoroalkoxy-[1,4]-naphthoquinones 1-3, 7 are reduced more easily than 2,3-bis (polyfluoroalkoxy)-[1,4]-naphthoquinones 4-6 (ΔЕ1 = 50 мВ). In turn, the reduction potential of non-fluorinated [1,4]-naphthoquinones 8,9 is greater than that of fluorinated compounds 1-7.
Cathodic reduction potentials of fluorine-containing [1,4]-naphthoquinones 1-7 do not depend on the length of fluoroalkyl substituents.
As a result of one-electron reduction 1-7 at low cathodic potentials, the stable radical anions are formed, which suggests their high oxidizing ability.
Experimental part
NMR and 19F spectra of CDCl3 were recorded via Bruker Avance-400 spectrometer with operating frequencies of 400 MHz and 376 MHz, respectively. The chemical shifts in 1H NMR spectra are given in δ (ppm) scale relative to TMS (as internal standard), in 19F NMR - relative to CFCl3 (as external standard). Spin-spin coupling constants are given in Hz. Rf of compounds were determined by TLC method using Merck TLC Silica gel 60 F254 plates. Electron impact mass spectra were obtained via FINNIGAN POLARIS Q spectrometer at 70 eV and at ion chamber temperature of 250°C.
The elemental analysis of compounds was carried out in the laboratory of elemental analysis of Institute of Ecological Studies RAS.
Cyclic voltammograms were recorded using IPC-Pro M potentiostat. The potential sweep rate was 200 mV/s. The concentration of compounds under study is 2.10-3 M.
Compounds 1-3 and 4-6 for studying of reduction potentials were obtained as described in [8].
2-Chloro-3-(2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9-hexadecafluorononyloxy)-[1,4]-naphthoquinone (7)
In a glass round-bottom flask equipped with a magnetic stirrer with heating and a reflux condenser were placed 226 mg (1 mmol) of 2,3-dichloro-[1,4]-naphthoquinone, 432 mg (1 mmol) 2,2,3,3,4 , 4,5,5,6,6,7,7,8,8,8,9,9-hexadecafluorononyl alcohol, 2.5 ml of absolute DMF and 160 mg (1.5 mmol) of NEt3. The contents of this flask were stirred for 3 h at a temperature of 70-75°C. The reaction mixture was cooled and 15 ml of distilled water was added to it. The formed precipitate was filtered off, washed with water and dried on a glass filter to constant weight. 500 mg of compound 7 was obtained. Yield 80,4%, melting point 81-82C, Rf = 0,40 (chloroform-cyclohexane ratio = 2: 1).
1H NMR (CDCl3, δ, ppm, J/Hz): 8,16 (m, 1H, Ar), 8,11 (m, 1H, Ar), 7,80 (m, 2H, Ar) - ABCD system); 6.06 (tt, 1H, CF2H, 2JH-F = 52, 3JH-F = 4); 5.08 (t, 2H, OCH2, 3JH-F = 11).
19F NMR (CDCl3, δ, ppm, J/Hz): -120,59 (br. s, 2 F, CF2); -121,93 (br. s, 6 F, 3CF2); -123,00 (br. s, 2F, CF2); -123,31 (br. s,2F, CF2); 129,32 (br. s, 2F, CF2); 136,99 (br. s, 2F, CF2).
Mass spectrum, m/z, (%): 622 (9) [M] +, 603 (8), 221 (100), 191 (16), 157 (72), 123 (27), 101 (22), 76 (19), 51 (78), 18 (21).
Founded, %: C, 36,38; H, 0,98; F, 49,01. C19H7ClF16O3. Calculated, %: C, 36,65; H, 1,13; F, 48,82.
2-Chloro-3-ethoxy-[1,4]-naphthoquinone (8).
In a glass round-bottom flask equipped with a magnetic stirrer with heating and a reflux condenser were placed 226 mg (1 mmol) of 2,3-dichloro-[1,4]-naphthoquinone, 3 ml of absolute ethanol and 320 mg (3 mmol) of NEt3. The reaction mass was stirred at a temperature of 65-70°C for 1.5 h. Then it was cooled and diluted with 10 ml of water with vigorous stirring for 10 min. The formed precipitate was filtered, washed with water and dried on a glass filter (and then - on filter paper) to constant weight. 215 mg chromatographically and spectrally pure compound 8 was obtained. Yield 91,1%, melting point 96-97°C *, Rf = 0,46 (CHCl3).
1H NMR (CDCl3, δ, ppm, J/Hz): 8,12 (m, 1H, Ar), 8,07 (m, 1H, Ar), 7,74 (m, 2H, Ar) - ABCD-system), 4,63 (q, 2H, OCH2, 3JH-H = 8), 1,46 (t, 3H, CH3, 3JH-H = 8).
Founded, %: C, 60,74; H, 3,92. C12H9ClO3. Calculated, %: C, 60,90; H, 3,83.
2-Butoxy-3-chloro-[1,4]-naphthoquinone (9).
In a glass round-bottom flask equipped with a magnetic stirrer with heating and a reflux condenser were placed 226 mg (1 mmol) 2,3-dichloro-[1,4]-naphthoquinone, 320 mg (3 mmol) NET3 and 3 ml of anhydrous butanol. The reaction mass was stirred for 4 h at a temperature of 70-75°C. Then the excess of butanol was removed via rotary evaporator, and the residue was dissolved in 2 ml of ethanol. From the resulting solution, compound 9 was precipitated with 15 ml of water. The reaction product was extracted using cyclohexane (three times with 10 ml). The resulting solution was dried over anhydrous CaCl2 and passed through a silica gel layer (h = 10 mm, Ø = 20 mm). The solution was evaporated in vacuo to give 1,6 g (62%) of chromatographically pure compound 9 as a viscous oil, that after cooling in a refrigerator and mashing becomes a solid with melting point 30-31°C.
1H NMR (CDCl3, δ, ppm, J/Hz): 8,13 (m, 2H, Ar), 7,75 (m, 2H, Ar)-ABCD-system); 4,58 (br. s, 2H, OCH2), 1,79 (m, 2H, CH2), 1,53 (m, 2H, CH2), 0,98 (br. s, 3H, CH3).
Founded, %: C, 63,41; H, 5,09. C14H13ClO3. Calculated, %: C, 63,52; H, 4,95.
Electrochemical studies
The reduction potentials -E11/2 and -E21/2 of naphthoquinones 1-11 (see Table 1) were measured by cyclic voltammetry method. Electrochemical studies were carried out in a dry argon atmosphere in 0.1 M Bu4NPF6 solution in anhydrous DMF. The measurements were carried out using three-electrode electrochemical cell with undivided cathode and anode space. The potentials were measured relative to aqueous saturated calomel electrode (SCE), separated from test solution in the cell by a bridge filled with solution of supporting electrolyte. A platinum plate located in the cell served as auxiliary electrode. The butt end of glass-carbon rod sealed into glass (disk area was 2 mm2) was used as a main electrode.
Acknowledgments
This work was financially supported by the Ministry of Science and Higher Education of Russian Federation using the scientific equipment provided by Center for Molecular Structures Study INEOS RAS.
References
- K. Krohn, Anthracycline Chemistry and Biology II: Mode of Action, Clinical Aspects and New Drugs (Ser. Topics in Current Chemistry), Springer, 2008, 223 p.
- F. Arcamone, Doxorubicin. Anticancer Antibiotics. Medicinal Chemistry, (Series of Monograph) - London: Academic press, 1981, 378 p.
- Guzik, T.J., Korbut, R., Adamek-Guzik, T., Journal of Physiology and Pharmacology, 2003, 54(4), 469-487.
- Е.F.Bell, History of vitamin E in infant nutrition, Am. J. of Clinic. Nutrition, 1987, 46(1), 183‑186.
- Skulachev V. P., Energetics of biological membranes, Moscow: Nauka; 1989, р. 564. (in Russian)
- Zhen Hui Lim et al., J. Phys. Chem., 2013, 117(8), 2396-2402.
- D.Wahl, A Short History of Electrochemistry - Part 2, Galvanotechtnik, 2005, 96(8), 1820‑1828.
- Dyachenko V.I., Fluorine notes, 2021, 1(134), 7-8.
- T.Fujinaga, K.Izutsu, T.Nomura, J. Electroanal.Chem., 1971, 29, 203.
- Fieser, L. F., Brown, R. H., J. Am. Chem. Soc., 1949, 71, 3609.
- Santos, Maria M. M. et al., Bioorganic & Medicinal Chemistry Letters, 2010, 20(1), 193-195.
- Jin-Cherng Lien et al., Chem. Pharm. Bull., 2002, 50(5), 672-674.
- Lee, D.-M., Ko, J. H., & Lee, K.-I. Monatshefte Für Chemie, Chemical Monthly, 2007, 138(8), 741–746.
- Yap S.P., Yuen K.H., Wong J.W., J. Pharm.Pharmacol., 2001, 53, 67-71.
- Leonard S.W., Paterson E., Atkinson J.K. et al., Free Radic. Biol. Med., 2005, 38, 857-866.
ARTICLE INFO
Received 14 April 2021
Accepted 20 April 2021
Available online April 2021
Recommended for publication by PhD M. A. Manaenkova
Fluorine Notes, 2021, 135, 5-6