Received: April 2021
DOI 10.17677/fn20714807.2021.02.01
Fluorine Notes, 2021, 135, 1-2
DIRECTED SYNTHESIS OF COPOLYMERS BASED ON FLUORINE-CONTAINING STYRENE DERIVATIVES
Chekurov K.E., Barabanova A.I.
A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Moscow, Russia
e-mail: chekurov@polly.phys.msu.ru
Abstract: The review considers and analyzes the literature data on the directed synthesis of (co) polymers based on fluorine-containing styrene derivatives, published over the past 15 years.
Key words: RAFT-polymerization, copolymers, fluorostyrenes
Introduction
Fluorinated polymers have recently attracted the attention of researchers not only due to their thermal and chemical resistance, resistance to wear and aging, but also poor wettability in aqueous and organic media. Low surface energy and high values of contact angles of fluorine-containing polymers make them very promising for the creation of various coatings, films and other products capable of self-cleaning from inorganic and organic contaminants [1–4]. To create products with the required properties, fluorine-containing (co) polymers with precisely known structure and molecular weight (MW) characteristics are required.
Synthesis of fluorine-containing copolymers by reversible deactivation radical polymerization
For directional design of fluorine-containing (co) polymers, anionic polymerization and reversible-deactivation radical polymerization (RDRP) are used [5–9]. More recently, Nishimura et al. reported anionic polymerization of 2,3,4,5,6-pentafluorostyrene (PFS) initiated by s-BuLi [5], and anionic copolymerization of PPS and p-divinylbenzene initiated by i-Pr2NLi [6]. Currently, controlled synthesis of fluorinated (co) polymers is carried out mainly by methods of radical polymerization with reversible deactivation, such as radical polymerization under conditions of reversible inhibition in the presence of nitroxides (nitroxide-mediated polymerization, NMP), atom transfer radical polymerization (ATRP), radical addition-fragmentation transfer (RAFT) polymerization due to their greater simplicity and versatility [7–9]. According to the mechanism of polymerization with the participation of stable nitroxyl radicals, mainly 2,2,6,6-tetramethylpiperidine-N-sila (TEMPO), only fluorine-containing styrenes and acrylates are successfully polymerized at very high temperatures (110-150°C) [8].
RAFT polymerization is the most promising approach to the preparation of fluorine-containing (co) polymers, including block copolymers. RAFT polymerization is the simplest and most versatile method of controlled synthesis, while this process does not require such harsh conditions as living anionic polymerization (the presence of a small amount of moisture and other impurities is permissible), proceeds at relatively low temperatures, does not require the use of organometallic catalysts, and suitable for radical polymerization of monomers [10–14].
The mechanism of RAFT polymerization, in addition to the elementary reactions of initiation, growth, and chain termination, which are traditional for radical polymerization, includes reversible chain transfer reactions specific for the RAFT process:
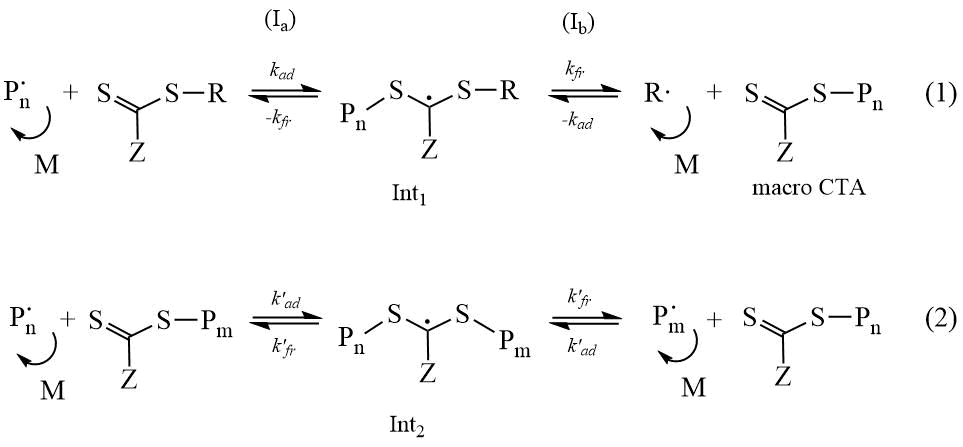
Scheme 1. Mechanism of RAFT polymerization.
The polymerization reaction is controlled by the addition of special compounds - chain transfer agent (CTA) to the system. The CTAs can behave as an ideal chain transmitter, reducing the Mn of the polymer and at the same time not affecting the polymerization rate [15]. Dithio- or trithio- compounds are usually used as CTAs (Scheme 2). The Z-group of the CTA is stabilizing, and the R-group is a leaving group of free radicals that must be able to re-initiate polymerization. The chemical nature of the Z and R functional groups determines both the kinetics of the process and the degree of control over key parameters of molecular weight distribution (MWD) and chain architecture.
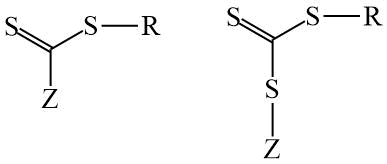
Scheme 2. General structural formulas of CTAs.
The efficiency of the CTA is determined, first, by the ratio of the rates of the possible decomposition processes of Int1 (reactions Ia and Ib): the easier and faster the leaving R-group is split off, the higher the efficiency of the CTA. Second, by the rate of addition of the Рn∙ radical to the CTA: the higher the value of ktr, the stronger the equilibrium of reaction (1) is shifted towards the formation of the polymer. If the equilibrium of reaction Iа is shifted towards the initial CTA, then RAFT polymerization is reduced to conventional radical polymerization.
To quantify the effectiveness of CTAs, the chain transfer constant to the CTA Сtr is used. If the consumption of the CTA and the monomer can be neglected, then the Сtr is calculated by the Mayo method [16].
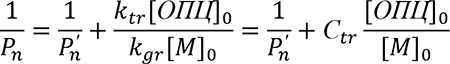 (1)
(1)
where Рп and Рп'- are the number average degree of polymerization in the presence and absence of the CTA, [М]0, [ОПЦ]0 - are the initial concentrations of the monomer and RAFT agent, respectively.
If the RAFT agent is consumed at the earliest conversions, the approach described in [10] is used ti estimate the Сtr:
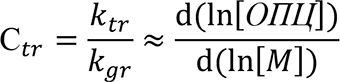 (2)
(2)
It is generally accepted that CTAs with Сtr >> 1 are effective.
RAFT polymerization of fluorine-containing styrene derivatives
Analysis of literature data showed that the most studied is the RAFT polymerization of fluorinated styrene (St) derivatives, including fluorostyrenes and their derivatives with substituents in the para position (Scheme 3), [17–28].
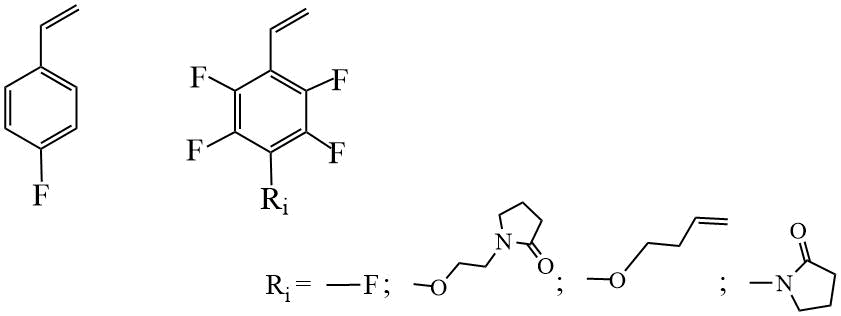
Scheme 3. Examples of styrenic monomers.
Examples of CTAs used in the RAFT polymerization of fluoromonomers are shown in Scheme 4.
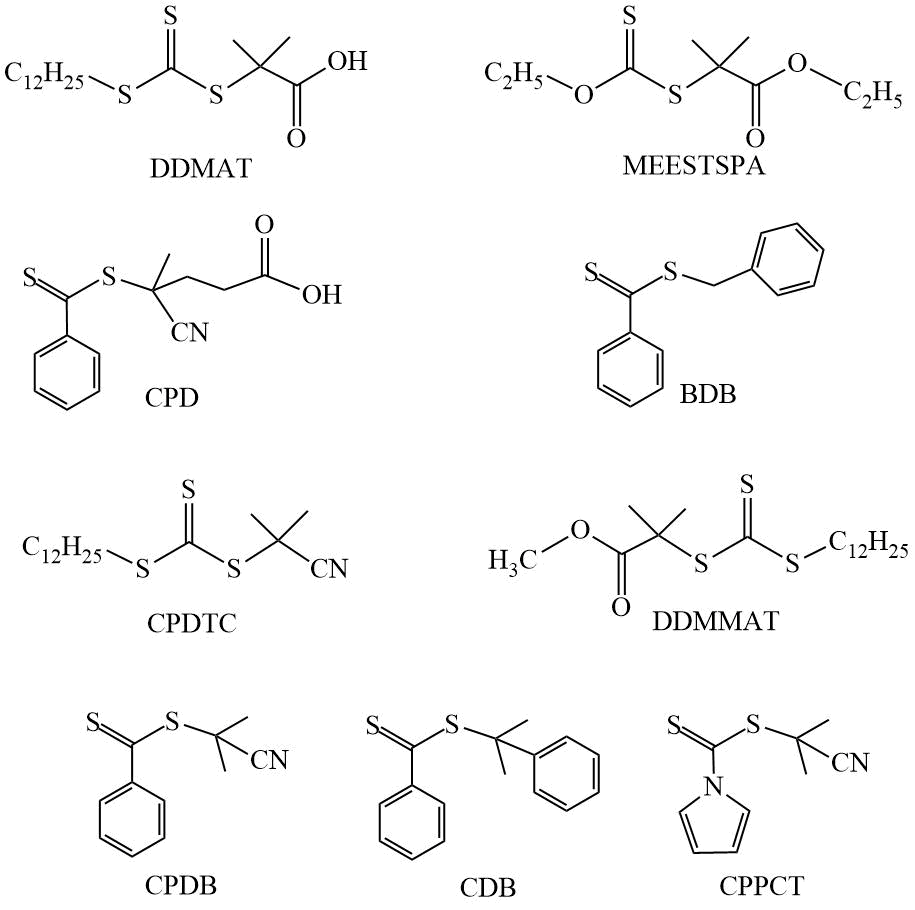
Scheme 4. Examples of CTAs.
One of the first studies of the RAFT polymerization of fluorostyrenes was carried out by Gudipati et al. [17]. They carried out a two-stage synthesis of amphiphilic DC based on glycidyl methacrylate (GMA) and PFS (Scheme 5) and showed that the polymerization of PFS in the presence of the polyglycidyl methacrylate-CTA proceeds according to the mechanism of RAFT polymerization with the formation of narrowly dispersed copolymers with a polydispersity index Mw/Mn < 1.2, with an increase in the reaction temperature, the polydispersity index of the DC increased: at 60°С Mw/Mn < 1.2, and at 80°C Mw/Mn < 1.5.
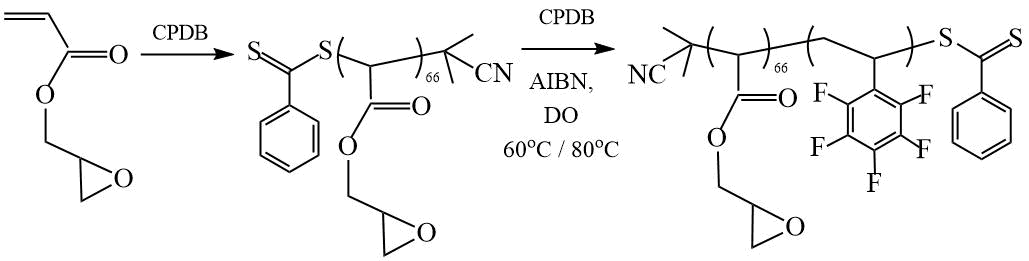
Scheme 5. Synthesis of DC (PGMA-PPFS) [17].
Ma et al. synthesized homopolymers of 4- (3'-butene-1'-oxy) -2,3,5,6-tetrafluorostyrene (BOFS) in the presence of S-1-dodecyl-S '- (α, α'-dimethyl-α' ' -methyl acetate) tritiocarbanate (DDMMAT) (Scheme 6) and AIBN in 2-butanone at 68°C and [BOFS] / [CTA] / [AIBN] = 50 / 1 / 0.1, alternating copolymers of BOFS with PFS also in 2-butanone at 68°С and [PFS] / [BOFS] / [CTA] / [AIBN] = 75 / 25 /1 / 0.1 [18]. Homopolymerization of BOFS and its copolymerization with PPS proceed according to a pseudo-living manner with the formation of narrowly dispersed polymers (Mw/Mn < 1.2 at the conversion below 50% and Mw/Mn = 1.35 at the conversion of 63%) and alternating copolymers (Mw/Mn = 1.19). The authors of [18] also synthesized narrowly dispersed DC based on PFS and BOFS using a copolymer of St and maleic anhydride (MAn) obtained by copolymerization in the presence of DDMMAT as a CTA.
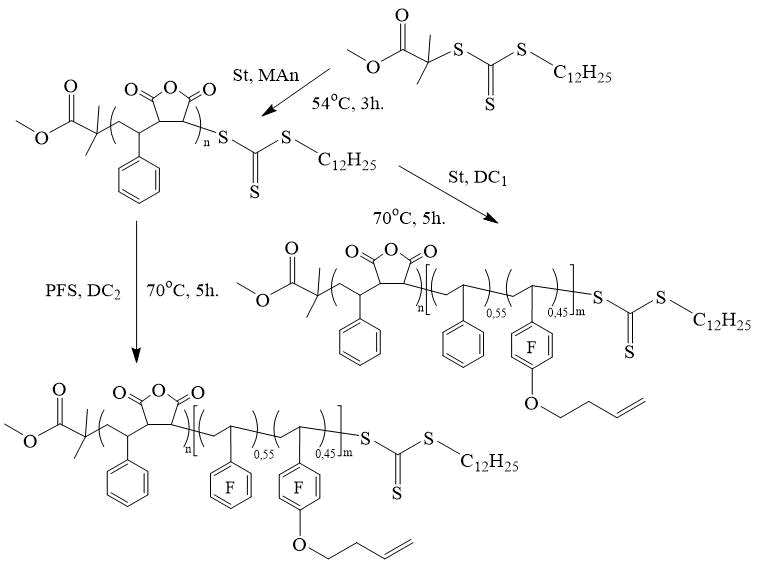
Scheme 6. Synthesis of copolymers based on PFS and BOFS [18].
The work of the same authors [19] reports on the synthesis of amphiphilic fluorine-containing block copolymers based on fluorostyrenes: 4- (3'-pyrrolidone-1'-oxy) -2,3,5,6-tetrafluorostyrene (POFS) and BOFS (Scheme 7). Coatings made from these block copolymers have been shown to be highly effective in protecting marine crafts from algae and shell growth.
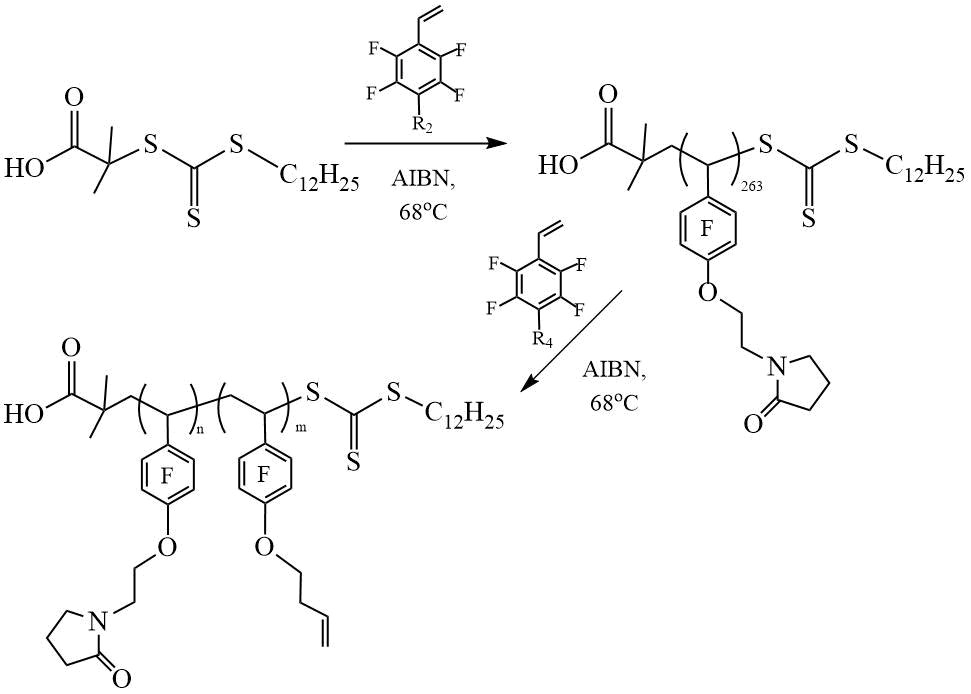
Scheme 7. Synthesis of DC (PPOFS-PBOFS) [19].
In [20], PPFS and high molecular weight DCs based on PFS and hydrophilic comonomers (methacrylate derivatives, 4-hydroxystyrene, 4-vinylpyridine) were obtained by polymerization of PFS initiated by AIBN and DDMAT as CTA, and copolymerization of PFS with hydrophilic comonomers in the presence of DDMAT and CPDB. It was found that homopolymerization of PFS, initiated by DDMAT, in polar DMF proceeds with a higher yield than in less polar anisole under the same conditions, while the experimentally determined Mn (GPC) values are close to theoretical values, and the polydispersity indices Mw/Mn are 1.09 ( in anisole) and 1.10 (in DMF). DCs of PFS with hydrophilic comonomers were synthesized starting from hydrophilic polymer blocks obtained by RAFT polymerization of the corresponding comonomers in the presence of DDMAT and CPDB.
In [21], a successful RAFT polymerization of St with PFS at [St] / [PFS] = 50/50 mol% in the presence of three CTAs: DDMAT, cyano-4 (thiobenzoylthio) pentanoic acid (CPD), and 2-cyano-2-propyl-1-pyrrolecarbodithioate (CPPCT) in 1,4-dioxane at 65°С with the formation of narrowly dispersed alternating copolymers with Mw/Mn = 1.31, 1.17, and 1.19, respectively has been reported. The most effective CTAs for this system are DDMAT and CPD.
More recently, Rowe et al. synthesized narrowly dispersed (Mw/Mn ≤ 1.26) alternating copolymers of PFS with N-phenylmaleimide with Mn = 9.8 ÷ 25 kDa copolymerization initiated by AIBN in the presence of DDMAT [22].
Zhu et al. developed anion-exchange membranes based on fluorine-containing DCs [23]. Narrowly dispersed DCs (Mw/Mn = 1.11 ÷ 1.31) were obtained by RAFT polymerization of 4-fluorostyrene (4-FS) and PFS with the initial macroCTA based on vinylbenzyl chloride (VBC) synthesized by polymerization of VBC in the presence of DDMAT (Scheme 8). The reaction was carried out at the ratio [mCTA] / [AIBN] = 10 / 1 and 5 / 1.
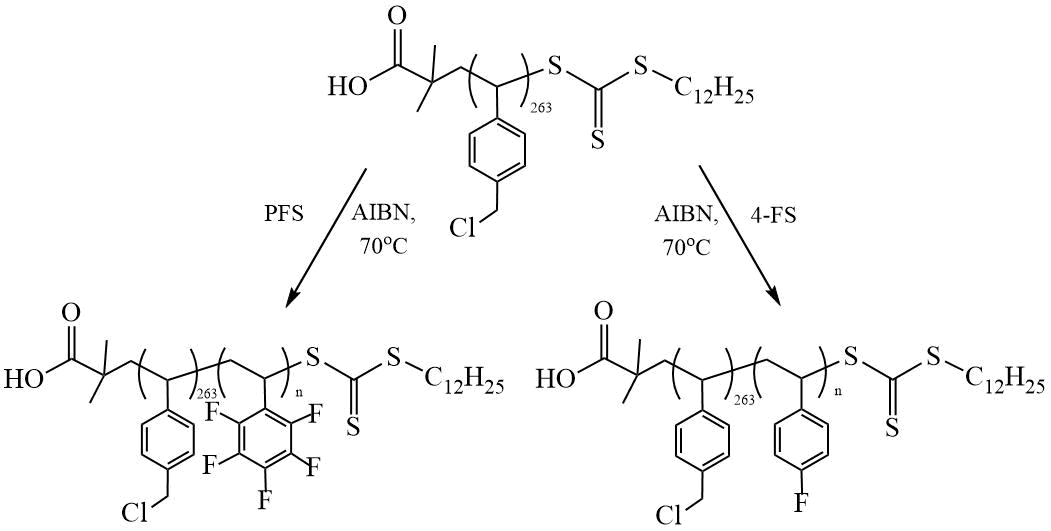
Scheme 8. Synthesis of DC (PVBC-PFS) and (PVBC-P(4-FS)) [23].
We recently investigated the RAFT polymerization of PFS and 2-hydroxyethyl methacrylate (HEMA) in the presence of CPDB [24] (Scheme 9). During the polymerization of PFS in the presence of the PHEMA-CTA at [PHEMA-CTA] / [AIBN] = 2, products are formed containing both DC and an insignificant amount of PPFS obtained by uncontrolled radical polymerization of PFS. With an increase in the [PHEMA-CTA] / [AIBN] ratio to 5, only DC is formed. Synthesized DCs containing less than 40% PFS units, chemically bonded to the surface of nylon fabric, improve its hydrophobic and oleophobic properties better than PPFS, which forms a defective fabric coating. It has been shown that nylon fabric treated with DC has higher contact angles (CA) of wetting with water and diiodomethane (DI) θН2О = 125 3 and θCH2I2 = 101 5 than PPFS coatings (θН2О = 102 2 and θCH2I2 = 74 1).
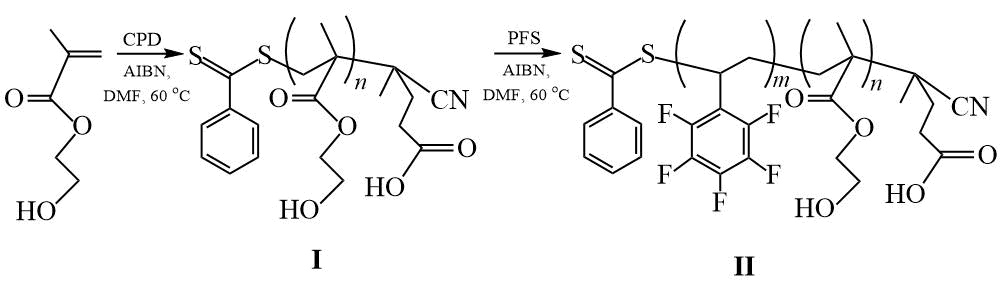
Scheme 9. Synthesis of DC (PHEMA-PPFS) [24].
In [25], the polymerization of PFS in the presence of the low molecular weight CTA CPDB was studied for the first time. The homopolymerization reaction of PFS was carried out in DMF at a molar ratio of [PFS] / [CPDB] / [AIBN] = 740 / 1.9 / 1 at 60°C. It is shown that the reaction proceeds according to the mechanism of the RAFT process with the formation of narrowly dispersed polymers with Mn = 11.9 ÷ 29.3 × 103 g / mol and Mw/Mn = 1.17 ÷ 1.41. This work also reports on the synthesis of DCs based on PFS and HEMA and on the study of the surface properties of fabric coatings from synthesized DCs. The CA values of wetting fabric coatings from the obtained DCs with water and DI are: θН2О = 120 6 and θCH2I2 = 93 2, which also exceeds the analogous values for PPFS.
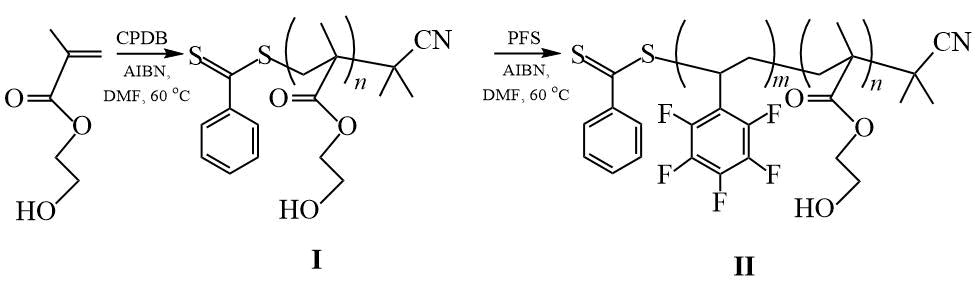
Scheme 10. Synthesis of DC (PHEMA-PPFS) [25].
We also investigated the kinetics of PFS homopolymerization in the presence of CPDB by 1Н NMR spectroscopy [26], and for the first time determined the chain transfer constant Сtr, which characterizes the efficiency of the CTA. For this, we investigated the radical polymerization of PFS in the presence of CPDB at the earliest stages of the process using 1Н NMR spectroscopy. It was found that during the polymerization of PFS in DMF-d7 at 50°C, CPDB is completely consumed in the early stages at q(PFS) = 0.83% and is an effective CTA with Сtr = 77.4 2.2.
In [27], a study of the polymerization of PFS in the presence of PHEMA with a terminal dithiobenzoate group as a CTA was reported. Monitoring of the reaction in DMF-d7 using in situ 1Н NMR spectroscopy showed that the reaction proceeds practically without an induction period at a higher rate than polymerization of PFS in the presence of a low molecular weight CTA. A possible reason for such kinetic regularities is the self-association of diblock copolymer macromolecules during polymerization, which is confirmed by the results of DLS. As the PPFS block is formed and the amount of PFS decreases, the microphase separation of the system begins with the formation of micelle-like aggregates with a hydrophobic PPFS core. Solubilization of PFS within such aggregates leads to an increase in the local concentration of monomer in the reaction zone and, as a consequence, to a higher polymerization rate compared to polymerization in the presence of CPDB.
The work [28] is devoted to the study of the polymerization of dimethylacrylamide (DMA) and PFS in the presence of Ethylsulfanylthiocarbonylsulfanylpropionic acid methyl ester (MEESTSPA) (Scheme 11), and to the study of the effect of synthesis conditions on the morphology of nanotubes formed as a result of self-assembly in the process of amphiphilic copolymers.
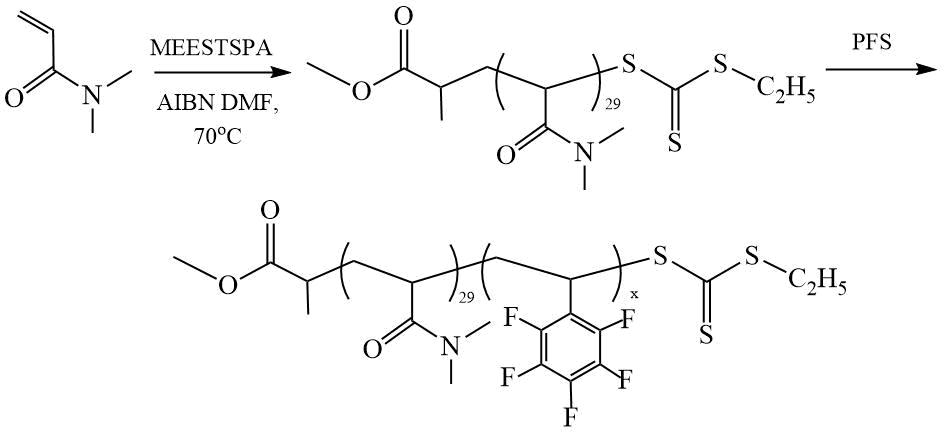
Scheme 11. Synthesis of DC (PDMA-PPFS) [28].
The authors investigated the effect of the reaction temperature (from 40 to 80°C) and the nature of the solvent (ethanol and its mixtures with 5-20% co-solvents such as DMF, toluene, dioxane, and THF) on the DCs ability to self-assemble, which leads to the formation of nanotubes. It was found that in ethanol at 70°C, nanotubes with a rough surface from copolymers with PnPFS = 180 ÷ 200 are formed. The addition of 5% co-solvents leads to a change in the morphology of nanotubes, for example, when 5% DMF is added, nanotubes with the smoothest surface are obtained. In a mixed solvent, the formation of nanotubes occurs at a reaction temperature that is below or close to the solvated glass transition temperature (Tsg), when the core-forming block has a limited degree of mobility, and one-dimensional fusion of vesicles becomes preferable.
Conclusion
Thus, an analysis of the literature has shown that the topic of RAFT polymerization of fluorinated styrene derivatives is a fairly young, but very promising field in the science of high molecular weight compounds. As can be seen from the review, fluorostyrenes are successfully polymerized by the mechanism of RAFT polymerization, and the range of agents used in the synthesis of RAFT agents, although small, is steadily expanding. It is expected that in the future the RAFT polymerization of fluorine-containing styrenes will develop, since there is a need to create new polymeric materials with properties that favorably distinguish fluorine-containing polymers from others.
Acknowledgement
The work was supported by the Ministry of Science and Higher Education of the Russian Federation.
References
- 1. Nishino T., Meguro M., Nakamae K., Matsushita M., and Ueda Y., The lowest surface free energy based on -CF3 alignment, Langmuir, 1999, 15(13), 4321-4323.
- Privett B.J., Youn J., Hong S.A., Lee J., Han J., Shin J.H., Schoenfisch M.H., Antibacterial fluorinated silica colloid superhydrophobic surfaces, Langmuir, 2011, 27(15), 9597-9601.
- Kondratenko M. S., Anisenko S. A., Elmanovich I. V., Stakhanov A. I., Gallyamov M. O., Khokhlov A. R., Hydrophobic properties of thin films of comb-shaped perfluorohexylethyl methacrylate-polydimethylsiloxane copolymers deposited from supercritical carbon dioxide solutions, Polym. Sci. - Ser. A, 2018, 60(4), 451-458.
- Zefirov V.V., Lubimtsev N.A., Stakhanov A.I. , Elmanovich I.V., Kondratenko M.S., Lokshin B.V., Gallyamov M.O., Khokhlov A.R., Durable crosslinked omniphobic coatings on textiles via supercritical carbon dioxide deposition, J. Supercrit. Fluids, 2017, 133, 30-37.
- Nishimura S., Nagai A., Takahashi A., Narita T., Hamana H., Anionic polymerization of 2,3,4,5,6-pentafluorostyrene, Polym. J., 1990, 22( 2), 171-174.
- Nishimura S., Nagai A., Takahashi A., Narita T., Anionic copolymerization, J. Polym. Sci. Part A: Polym. Chem., 1993, 31(1), 135-139.
- Souzy R., Ameduri B., Boutevin B., Synthesis and (co)polymerization of monofluoro, difluoro, trifluorostyrene and ((trifluorovinyl)oxy)benzene, Prog. Polym. Sci., 2004, 29(3), 75-106.
- Natanya M.L. Hansen, Jankova K., Hvilsted S., Fluoropolymer materials and architectures prepared by controlled radical polymerizations, Eur. Polym. J., 2007, 43(2), 255-293.
- Bruno A., Controlled radical (Co)polymerization of fluoromonomers, Macromolecules, 2010, 43(24), 10163-10184.
- Chiefari J., Mayadunne R.T.A., Moad C.L., Moad G., Rizzardo E., Postma A., Skidmore M.A., Thang S.H., Thiocarbonylthio compounds (S=C(Z)S-R) in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT polymerization). Effect of the activating Group Z, Macromolecules, 2003, 36(7), 2273-2283.
- Chong Y-K., Krstina J. Le T.P.T., Moad G., Postma A., Rizzardo E., Thang S.H., Thiocarbonylthio compounds [S=C(Ph)S-R) in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT polymerization). Role of the free-radical leaving group (R), Macromolecules, 2003, 36(7), 2256-2272.
- Moad G., Rizzardo E., Thang S.H., Living radical polymerization by the RAFT process A second update, Aust. J. Chem., 2009, 62, 1402-1472.
- C. Barner-Kowollik, Handbook of RAFT polymerization, Weinheim: Wiley-VCHH Verlag Gmbh & Co., 2008, p. 548.
- Chernikova E.V., Sivtsov E.V., Reversible addition-fragmentation chain-transfer polymerization: Fundamentals and use in practice, Polym. Sci., Ser. B, 2017, 59(2), 117-146.
- Barner-Kowollik C., Quinn J.F., Nguyen T.L.U., Heuts J.P.A., Davis T.P., Kinetic investigations of reversible addition fragmentation chain transfer polymerizations: Cumyl phenyldithioacetate mediated homopolymerizations of styrene and methyl methacrylate, Macromolecules, 2001, 34(22), 7849-7857.
- Mayo F. R., Chain transfer in the polymerization of styrene: the reaction of solvents with free radicals, J. Am. Chem. Soc., 1943, 65(12), 2324-2329.
- Gudipati C. S., Tan M. B. H., Hussain H., Liu Y., He C., Davis T.P., Synthesis of poly(glycidyl methacrylate)-block-poly(pentafluorostyrene) by RAFT: Precursor to novel amphiphilic poly(glyceryl methacrylate)-block- poly(pentafluorostyrene), Macromol. Rapid Commun., 2008, 29(23), 1902-1907.
- Ma J., Cheng C., Sun G., Wooley K.L., Well-defined polymers bearing pendent alkene functionalities via selective RAFT polymerization, Macromolecules, 2008, 41(23), 9080-9089.
- Ma J., Bartels J. W., Li Z., Zhang K., Cheng C., Wooley K. L., Synthesis and solution-state assembly or bulk state thiol-ene crosslinking of pyrrolidinone- and alkene-functionalized amphiphilic block fluorocopolymers: From functional nanoparticles to anti-fouling coatings // Aust. J. Chem., 2010, 63(8), 1159-1163.
- Riedel M., Stadermann J., Komber H., Simon F., Voit B., Synthesis, post-modification and self-assembled thin films of pentafluorostyrene containing block copolymers, Eur. Polym. J., 2011, 47(4), 675-684.
- Brummelhuis N. T., Weck M., RAFT polymerization of alternating styrene-pentafluorostyrene copolymers, J. Polym. Sci. Part A Polym. Chem., 2014, 52(11), 1555-1559.
- Rowe M., Teo G.H., Horne J., Al-Khayat O., Neto C., Thickett S. C., High glass transition temperature fluoropolymers for hydrophobic surface coatings via RAFT copolymerization, Aust. J. Chem., 2016, 69(7), 725-734.
- Zhu M., Zhang X., Wang Y., Wu Y., Wang H., Zhang M., Chen Q., Shen Z., Li N., Novel anion exchange membranes based on quaternized diblock copolystyrene containing a fluorinated hydrophobic block, J. Memb. Sci., 2018, 554, 264-273.
- Chekurov K.E., Barabanova A.I., Blagodatskhikh I.V., Peregudov A.S., Khokhlov A.R., Synthesis and repellent properties of fluorinated diblock-copolymers, Fluorine Notes, 2019, 2(123), 1-2.
- Chekurov K.E., Barabanova A.I., Blagodatskikh I.V., Lokshin B.V., Peregudov A.S., Abramchuk S.S., Khokhlov A.R., Synthesis and self-assembling of amphiphilic diblock copolymers of 2,3,4,5,6-pentafluorostyrene, Dokl. Chem., 2019, 484(2), 33-36.
- Chekurov K.E., Barabanova A.I., Peregudov A.S., Khokhlov A.R., The investigation of the polymerization of 2,3,4,5,6-pentafluorostyrene in the presence of 2-cyano-2-propyl-dithiobenzoate by 1Н NMR-spectroscopy Fluorine Notes, 2019, 3(124), 1-2.
- Chekurov K.E., Barabanova A.I., Blagodatskikh I.V., Muranov A.V., Laptinskaya T.V., Peregudov A.S., Khokhlov A.R., Study of the Polymerization of 2,3,4,5,6-Pentafluorostyrene in the Presence of Poly-2-hydroxyethyl Methacrylate Chain Transfer Agent, Dokl. Chem., 2020, 490(2), 27-31.
- Lv F., An Z., Wu P., What determines the formation of block copolymer nanotubes?, Macromolecules, 2020, 53(1), 367-373.
ARTICLE INFO
Received 07 April 2021
Accepted 15 April 2021
Available online April 2021
Recommended for publication by PhD V. Don
Fluorine Notes, 2021, 135, 1-2
