Received: February 2021
DOI 10.17677/fn20714807.2021.01.04
Fluorine Notes, 2021, 134, 7-8
Synthesis of 2-chloro-3-polyfluoroalkoxy- and 2,3-bis (polyfluoralkoxy)-[1,4]-naphthoquinones
V.I. Dyachenko
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991, GSP-1, Moscow, B-334, Vavilov St. 28
e-mail: vic-d.60@mail.ru
Abstract: For the first time the reaction of polyfluorinated aliphatic alcohols 2а-d with 2,3-dichloro-[1,4]-naphthoquinone 1 has been studied. It has been shown that 2a-d in anhydrous dimethylformamide in the presence of NEt3 easily reacts with 1, forming 2-polyfluoroalkoxy-3-chloro-[1,4]-naphthoquinones 3a-c with a yield of 83-93%. An excess of alcohol 2a-d under these conditions accelerates the reaction rate and does not lead to substitution of second chlorine atom. The reaction of 1 with carbinols 2а-d in the presence of anhydrous К2СО3 at a temperature of 50‑55С allows to obtain 2,3-bis-polyfluoroalkoxy [1,4]-naphthoquinones 4a-c with a yield of 74‑76%.
Keywords: polyfluorinated alcohols, 2,3-dichloro-[1,4]-naphthoquinone, 2-polyfluoroalkoxy-3-chloro-[1,4]-naphthoquinones, potash, 2,3-bis(polyfluoroalkoxy)-[1,4]-naphthoquinones
Introduction
[1,4]-naphthoquinones are one of the most important classes of organic compounds with antioxidant properties [1]. 2-methylnaphthoquinone is a part of vitamin molecule K1 (see Fig. 1), which regulates the normal level of blood coagulation, as well as the exchange of Са+2 in bone tissue [2]. Its excess leads to increase in blood coagulation, which complicates the treatment of heart attack, cerebrovascular accident and other diseases associated with thrombus formation [3]. The synthetic derivatives of [1,4]-naphthoquinone are used as dyes for fabrics and reagents for photometric determination of metals, additives to polymers to extend its service life [4,5]. Condensed [1,4]-naphthoquinones doxorubicin and daunomycin are widely used in the treatment of oncological diseases [6,7]. In order to search for new anticancer drugs, the synthesis of polycyclic derivatives of [1,4]-naphthoquinones containing a СF3-group is being undertaken [8,9]. The synthesis of basic fluorine-containing [1,4]-naphthoquinones – the potential precursors for synthesis of antioxidants and detectors for analytical chemistry, as well as compounds with biological activity [10-11] is of considerable interest. In this regard, the search for new synthetic procedures of polyfluoroalkyl-containing [1,4]-naphthoquinones is of great importance.
The reaction of aliphatic alcohols with 2,3-dichloro-[1,4]-naphthoquinone has been well studied [12-14]. As a result of this reaction, that carried out, as a rule, in polar solvents and in the presence of carbonates or alcoholates of alkali metals, nucleophilic substitution of chlorine for alcoholate ion occurs with the formation of mono- or bis (alkoxy)-[1,4]-naphthoquinones. Depending on the ratio of reactants and reaction conditions, one or two halogen atoms are replaced.
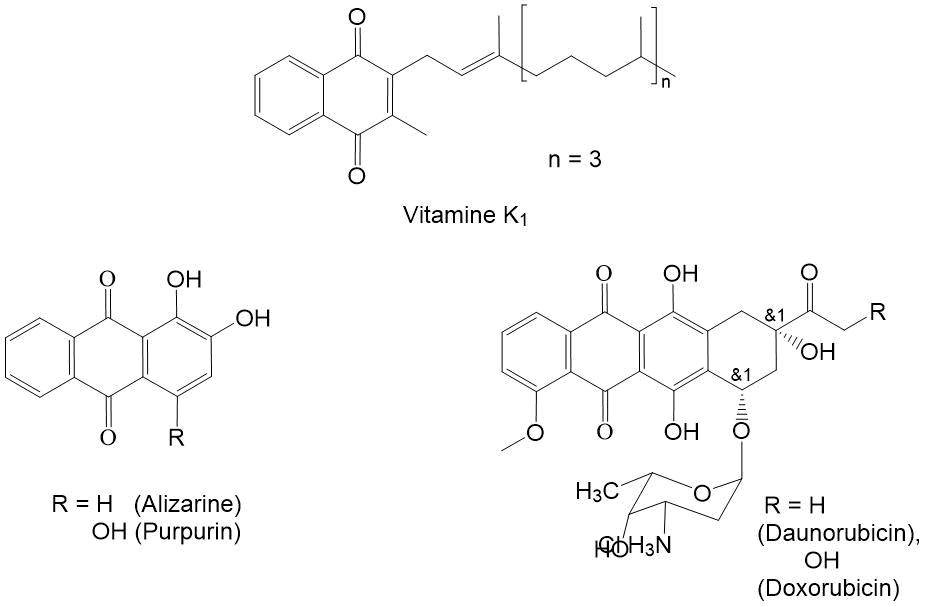
Figure 1. [1,4]-Naphthoquinones used in medicine.
At the same time, the reaction of 2,3-dichloro-[1,4]-naphthoquinones with fluorine-containing alcohols as a platform for the synthesis of polyfluoroalkoxy-[1,4]-naphthoquinones has not been studied.
The authors showed that primary polyfluorinated alcohols 2а-с in anhydrous dimethylformamide (DMF) in the presence of triethylamine at 50-55С readily reacts with 2,3-dichloro-[1,4]-naphthoquinone to form the corresponding 2-chloro-3-(polyfluoroalkoxy) -[1,4]-naphthoquinones (3а-с) with yield of 83-93% (see Scheme 1).
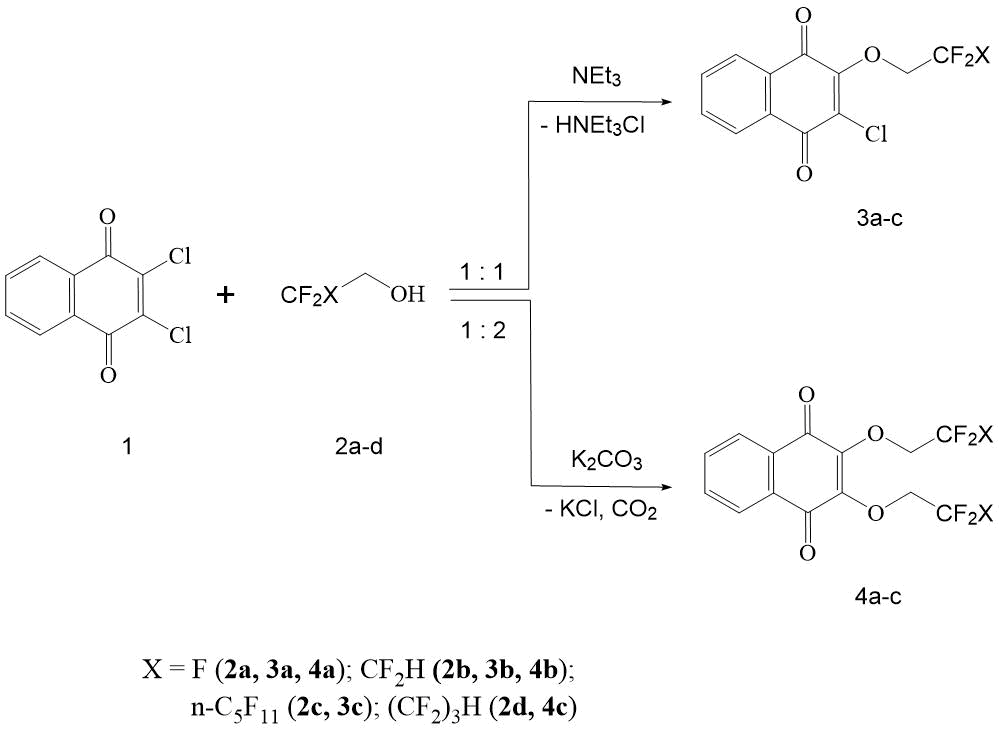
Scheme 1. Formation of 2-chloro-3-(polyfluoroalkoxy)-[1,4]-naphthoquinones and 2,3-bis (polyfluoroethoxy)-[1,4]-naphthoquinones.
It should be noted that this reaction takes place already at 20°C, and an increase in temperature only accelerates it in time. An excess of polyfluorocarbinol, as well as NEt3, accelerates the formation of naphthoquinones 3a-c. So, with a 3-fold excess of NEt3 the synthesis time of 3b (in comparison with 3a) is significantly reduced (see section “Experimental part”). It is characteristic that under these conditions the reaction proceeds unambiguously and is not complicated by formation of by-products, as well as of substitution products of the second chlorine atom in naphthoquinone 1.
Thus, the determined conditions of reaction make it possible to selectively obtain the monochlorine substitution products 3а-с with a high yield.
To formation of 2,3-bis-(polyfluoroethoxy)-[1,4]-naphthoquinones (4а-с) in reaction 1 and polyfluorinated alcohols 2а,d, catalysts that are more basic than NЕt3, as HCl acceptors, are required. In the case of reaction of 2,3-dichloro-[1,4]-naphthoquinone with non-fluorinated carbinols, the best yields are obtained by carrying out the reaction in alcohol in the presence of corresponding alcoholate [12]. Good results have also been obtained in case when these transformations are carried out in DMSO or CH3CN and in the presence of alkali metal carbonates [13].
The authors showed that 1 in anhydrous DMF in the presence of K2CO3 at 50-55°С readily reacts with polyfluorinated alcohols 2а,b,d, forming the corresponding 2-chloro-3-(polyfluoroalkoxy)-[1,4]-naphthoquinones 4а-с with yield of 74-76%. The reaction is carried out in heterogeneous conditions with vigorous stirring for 2.5-3 h.
In most cases, the isolation and purification of 3a-c and 4a-c is distinguished by its simplicity, which consists only in diluting the reaction mixture with water and filtering the precipitated reaction product.
It is interesting to note that with increasing in atomic mass (in a number of fluorine atoms) of polyfluoroalkoxy substituents in 2,3-position of [1,4]-naphthoquinones 4а-c, their melting point decreases. So, 4а melts at 81-82°С, 4b - at 42-43°С; 4c at 20°С is the thin oil.
The reactivity of chlorine atom in compounds (as well as the presence of carbonyl groups) localize the mono- and bis(polyfluoroalkoxy)-[1,4]-naphthoquinones 3а-с and 4а-с synthesized by us as a promising synthons for further synthetic transformations, as well as for antioxidant modification (bio)polymers.
Experimental part
NMR 1H and 19F spectra were recorded in СDCl3 via Bruker Avance 400 spectrometer at operating frequencies of 400 MHz and 376 MHz, respectively. The chemical shifts in 1H NMR spectra are given in δ (ppm) scale relative to TMS as internal standard; in 19F NMR spectra of compounds 3а, 3b and 4а are given in ppm relative to СF3CO2H as external standard; in spectra of compounds 3c, 4b, 4с - relative to CFCl3 as external standard. Spin-spin coupling constants are given in Hz. Rf for compounds were determined by TLC method via Merck TLC Silica gel 60 F254 plates.
Electron impact mass spectra were obtained via FINNIGAN POLARIS Q spectrometer at 70 eV and ion chamber temperature of 250°C.
The elemental analysis of compounds was carried out in the laboratory of elemental analysis in INEOS RAS.
2-chloro-3-(2,2,2-trifluoroethoxy)-[1,4]-naphthoquinone (3а)
In a glass flask equipped a reflux condenser with calcium chloride tube and a magnetic stirrer was placed 226 mg (1 mmol) of 2,3-dichloro-[1,4]naphthoquinone 1, 150 mg (1.5 mmol) of 2,2,2-trifluoroethanol 2a and 1.2 ml of anhydrous dimethylformamide (DMF), then 150 mg (1.5 mol) of triethylamine was added (dropwise and with stirring) to the reaction mass. The reaction temperature was raised to 50-55°C and kept under these conditions for 1.5 h. The reaction mixture was cooled to 20°C and diluted with 10 ml of water. The formed precipitate was filtered off, washed with water and dried to constant weight at first on a glass filter and then - in vacuum over Р2О5. As a result 260 mg of chromatographically and spectrally pure compound 3а was obtained with yield of 89,7%, melting point 105-106°C, Rf = 0,43 (СHCl3).
1H NMR (СDСl3, δ, ppm, J/Hz): 8.18 (m, 1H, Ar), 8.16 (m, 1H, Ar), 7.81 (m, 2H, Ar) - ABCD system; 4.93 (q, 2Н, OCH2, 3JH-F = 8).
19F NMR (CDCl3, δ, ppm, J /Hz): 2,77 (s, 3F, CF3).
Mass spectrum, m/z, (%): 290 (43) [M]+, 270 (10), 157 (75), 151 (53), 129 (20), 123 (83), 104 (27), 83 (25), 76 (38), 64 (15), 50 (33), 18 (53).
Founded, %: C, 50.07; H, 1,98; F, 19,29. C12H6ClF3O3. Calculated, %: C, 49,59; H, 2,08; F, 19,61.
2-chloro-3-(2,2,3,3-tetrafluoropropoxy)-[1,4]-naphthoquinone (3b)
In a glass flask, equipped as in synthesis 3a, was placed 226 mg (1 mmol) of 2,3-dichloro-[1,4]naphthoquinone 1, 396 mg (3 mmol) of 2,2,3,3-tetrafluoropropanol 2b and 1,2 ml of anhydrous dimethylformamide (DMF), then about 300 mg (3 mmol) of triethylamine was added (dropwise and with stirring) to the reaction mass at 20°C. The reaction temperature was raised to 50-55°C and stirred for 0.5 h. The reaction mixture was cooled to 20°C and diluted with 10 ml of water. The formed precipitate was filtered off, washed with water and dried to constant weight at first on a paper filter and then - in a vacuum over Р2О5. As a result 300 mg of chromatographically and spectrally pure compound 3b was obtained, with a yield of 93.2%, melting point 119-120°C, Rf = 0,40 (СHСl3).
1H NMR (CDCl3, δ, ppm, J /Hz): 8,19 (m, 1H, Ar), 8,13 (s, 1H, Ar), 7,81 (m, 2H, Ar) - ABCD system; 6.21 (tt, 1H, СF2H, 2JH-F = 52, 3JH-F = 4); 4,94 (t, 2H, OCH2, 3JH-F=11).
19F NMR (СDСl3, δ, ppm, J /Hz): -48.21 (s, 2F, CF2); -61,66 (s, 2F, CF2). Founded, %: C, 48,58; H, 2,08; F, 23,22. C13H7ClF4NO3. Calculated, %: C, 48,40; H, 2,19; F, 23,55.
2-chloro-3-(2,2,3,3,4,4,5,5,6,6,7,7,7-tridecafluoroheptyloxy)-[1,4] naphthoquinone (3c)
During 3 h and according to synthesis method 3а from 226 mg (1 mmol) 2,3-dichloro-[1,4] naphthoquinone 1, 385 mg (1,1 mmol) 2,2,3,3,4,4,5,5,6,6,7,7,7-tridecafluoroheptanol 2с and 150 mg (1,5 mol) of triethylamine 450 mg was obtained the chromatographically and spectrally pure compound 3с, with a yield of 83,3%, melting point 82-83°С, Rf = 0,57 (СHСl3).
1Н NMR (СDСl3, δ, ppm, J/Hz): 8,19 (m, 1H, Ar), 8,13 (m, 1H, Ar), 7,82 (m, 2H, Ar) - ABCD system); 5,10 (t, 2H, OCH2, 3JH-F=11).
19F NMR (CDCl3, δ, ppm, J/Hz): -80,71 (t, 3 F, CF3, 3JF-F=11); -120.56 (td, 2 F, CF2, 3JF-Н = 11, 4JF-F = 4); -122,08 (m, 2F, CF2); -122,74 (m, 2F, CF2); -122,99 (m, 2F, CF2); -126,09 (t, 2F, CF2, 3JF-F = 15).
Founded, %: C, 38,13; H, 1,28; F, 46,04. C17H6ClF13O3. Calculated, %: C, 37,77; H, 1,12; F, 45,68.
2,3-bis-(2,2,2-trifluoroethoxy)-[1,4]-naphthoquinone (4а)
In a glass flask were placed 226 mg (1 mmol) of 2,3-dichloro-[1,4]naphthoquinone 1, 300 mg (3 mmol) of 2,2,2-trifluoroethanol 2a, 168 mg (3 mmol) of potash and 2,5 ml of anhydrous dimethylformamide (DMF), then the reaction mixture was stirred on a magnetic stirrer at 50-55°C for 2.5 h and the reaction mass was diluted with 10 ml of water. The formed precipitate was filtered off, washed with water and dried to constant weight at first on a glass filter and then - in a vacuum over Р2О5. As a result 270 mg of chromatographically and spectrally pure compound 4а was obtained, with yield of 76.3%, melting point 81-82 °C, Rf = 0.33 (СHСl3).
1H NMR (CDCl3, δ, ppm, J/Hz): 8,10 (dq, 2H, Ar); 7,78 (dq, 2H, Ar), ABCD-system; 4,78 (q, 4H, 2OCH2, 2J=8).
19F NMR (CDCl3, δ, ppm, J/Hz): 2,77 (s, 3F, CF3).
Founded, %: C, 47,63; H, 2,22; F, 31,82. C14H8F6O4. Calculated, %: C, 47,47; H, 2,28; F, 32,18.
2,3-bis-(2,2,3,3-tetrafluoropropoxy)-[1,4]-naphthoquinone (4b)
According to synthesis method 4а from 226 mg (1 mmol) 2,3-dichloro-[1,4] naphthoquinone 1, 395 mg (3 mmol) of ,2,3,3-tetrafluoropropanol 2b and 168 mg (3 mmol) of potash was obtained 210 mg of chromatographically and spectrally pure compound 4b. The resulting filtrate was extracted (3 x 10 ml) by ethyl acetate-cyclohexane mixture = 1:10. The organic layer was separated, dried by anhydrous potash, filtered through silica gel and boiled off to constant weight via rotary evaporator. An additional 100 mg of chromatographically pure solid compound 4b was obtained, with overall yield 74,2%, melting point 42-43°C, Rf = 0,34 (СHСl3).
1H NMR (CDCl3, δ, ppm, J/Hz): 8,09 (dq, 2H, Ar); 7,79 (dq, 2H, Ar), ABCD-system; 6,15 (tt, 2Н, 2 CF2H, 2JH-F = 64, 3JH-F = 4); 4,78 (t, 4H, ОСН2, 2JH-F =16).
19F NMR (CDCl3, δ, ppm, J/Hz): -125,40 (s, 2F, CF2); -138,86 (s, 2F, CF2).
Mass spectrum, m/z, (%): 418 (29) [M]+, 317 (74), 187 (54), 173 (100), 157 (19), 133 (36), 104 (98) 89 (28), 76 (68), 64 (16), 51 (77).
Founded, %: C, 45,88; H, 2,13; F, 35,98. C16H10F8O4. Calculated, %: C, 45,95; H, 2,41; F, 36,34.
2,3-bis- (2,2,3,3,4,4,5,5-octafluoropentyloxy)-[1,4]-naphthoquinone (4c)
During 3 h and according synthesis procedure 4a from 226 mg (1 mmol) of 2,3-dichloro[1,4]naphthoquinone 1, 580 mg (2.5 mmol) of 2,2,3,3,4,4,5,5-octafluoropentanol 2d and 168 mg (3 mmol) potash the compound 4с was obtained. The reaction mixture was diluted with water and extracted (3 x 15 ml) by ethyl acetate-cyclohexane mixture = 1:10. The organic layer was separated, dried by anhydrous potash, filtered through a layer of silica gel and boiled off via rotary evaporator at a water bath temperature of 90-95°C. 475 mg of compound 4c was obtained as a yellow thin oil, with yield of 76.8%, nD20 = 1,446, Rf = 0,38 (СHСl3).
1H NMR (CDCl3, δ, ppm, J/Hz): 8,95 (dq, 2H, Ar); 7,78 (dq, 2H, Ar), ABCD-system; 6,12 (tt, 2Н, 2CF2H, 2JH-F = 48, 3JH-F = 4); 4,93 (t, 4H, ОСН2, 2JH-F = 11).
19F NMR (CDCl3, δ, ppm, J /Hz): -121,06 (t, 2F, CF2, 3JF-F = 7); -125,55 (t, 2F, CF2, 3JF‑F = 7); -130,17 (m, 2F, CF2); -137,33 (m, 2F, CF2).
Founded, %: C, 39,15; H, 1,48; F, 48,91. C20H10F16O4. Calculated, %: C, 38,85; H, 1,63; F, 49,16.
Acknowledgments
This work was financially supported by the Ministry of Science and Higher Education of Russian Federation using scientific equipment of the Center of Molecules Structure Study of INEOS RAS.
Mass spectral studies were supported at the expense of grant No. 20-03-00468a from Russian Foundation for Basic Research.
References
- Thomson R.H., Naturally occurring quinones IV: recent advances. — London: Springer, 1997, 746 p.
- G. F. M. Ball, Vitamins: their role in the human body, Blackwell Science, 2004, 448 p.
- Maximilian Ackermann at al., Pulmonary vascular Endothelialitis, Trombosis and Angiogenesis in Covid-19, Nеw. Engl. J. Med., 2020, 383, 120-128.
- Pierce E., Histochemistry, trans. from Engl., M., 1962, 607 p.
- Burston M., Histochemistry of enzymes, trans. from Engl. M., 1965
- K. Krohn., Anthracycline Chemistry and Biology II: Mode of Action, Clinical Aspects and New Drugs (Ser. Topics in Current Chemistry) - Springer, 2008, 223 p.
- F. Arcamone., Doxorubicin. Anticancer Antibiotics., Medicinal Chemistry, (Series of Monograph) - London: Academic press, 1981, 378 p.
- Davydov, D. V. and Beletskaya, I. P., Russ. J. Organ. Chem., 2004, 40(1), 134-136.
- Sone, Toshihiko et al, PCT Int. Appl., 2015120304 (2015).
- (а) Zhang, Yan et al, Org. Lett., 2017, 19(6), 1302-1305; (b) Paul, Kamaldeep et al, Org. Lett., 2009, 11(20), 4728-4731.
- Lien, Jin-Cherng et al, Chem. Pharm.Bull., 2002, 50(5), 672-674.
- Dias, Gleiston G. et al, Chem. Comm. (Cambridge, United Kingdom), 2018, 54 (91), 12840.
- Monika Kadela-Tomanek at all., Molecules, 2017, 22, 447.
- Brandy, Yakini et al, Molecules, 2013, 18, 1973.
ARTICLE INFO
Received 12 February 2021
Accepted 15 February 2021
Available online February 2021
Recommended for publication by PhD M. Manaenkova
Fluorine Notes, 2021, 134, 7-8
