Received: January 2021
DOI 10.17677/fn20714807.2021.01.01
Fluorine Notes, 2021, 134, 1-2
SYNTHESIS OF β-NaYF4/Yb+3/Er+3 FLUORIDE NANOCRYSTALS AT HIGH PRESSURE
V.I. Sokolov1,2, I.M. Asharchuk1, E.N. Glazunova1, I. O. Goryachuk1, A.V. Lyubeshkin1
1Institute of Photonic Technologies, Federal Scientific Research Center "Crystallography and Photonics" RAS, 119333, Leninsky ave., 59, Moscow, Russiф
2Federal Research Center “Scientific Research Institute for System Research” RAS, 117218, Nakhimovsky ave., 36, bldg. 1, Moscow, Russia
Annotation: Fluoride nanocrystals of NaYF4/Yb+3/Er+3 have been synthesized in pure cubic (alpha-) and pure hexagonal (beta-) phases. The transition of α-nanoparticles in a solution of oleic acid and 1-octadecene in β-phase occurs at a temperature of 380 °C and a pressure of 10 atm. This transition is monitored by a change in photoluminescence spectrum of rare-earth elements Yb and Er upon pumping by laser radiation with a wavelength of 980 nm directly during synthesis (in situ). The resulting nanocrystals embedded in a polymer matrix can be used to create compact waveguide light amplifiers in the optical data transmission buses at optoelectronic printed circuit boards.
Keywords: nanoscale fluoride crystals, rare earth elements, photoluminescence, synthesis at high pressure.
Introduction
To date, fluoride nanocrystals NaYF4, NaLuF4 etc. doped with rare earth elements Yb, Er, Tm, Ce [1–4] are widely used both in biomedicine (see [5, 6] and references in these papers), as well as in technology (for example, to create three-dimensional displays [7], high-performance solar cells [8], up-converting visible-light lasers [9] and compact waveguide light amplifiers [10 - 13]). Fluoride nanocrystals (nanophosphors) can exist in the form of a cubic crystal lattice (so-called α-phase), or in the form of a hexagonal lattice (β-phase). The photoluminescence (PL) spectra of rare-earth ions in the NaYF4 nanocrystal differ significantly in case of a cubic or hexagonal phase [1 - 5, 14]. Thus, the efficiency of upconversion (i. e. conversion of IR pump radiation into green and red emission in visible-light spectrum) in nanocrystals of β-NaYF4/Yb+3/Er+3 is more than an order of magnitude higher than that in nanocrystals of α-NaYF4/Yb+3/Er+3 [2]; therefore, it is important to develop reliable synthesis methods for nanophosphors with a predetermined crystal structure.
This article reports on the transformation of fluoride nanocrystals of α-NaYF4/Yb+3/Er+3 in a solution of oleic acid and 1-octadecene at a pressure of 10 atm and a temperature of 380°C. To observe the formation of nanophosphors in β-phase in situ, the authors used the control of intensity and spectrum of their photoluminescence directly during their synthesis in a chemical reactor. The resulting nanoparticles of β-NaYF4/Yb+3/Er+3 are 1 - 2 μm in size and exhibit high PL in upconversion (within the spectral range of 475 - 725 nm) when pumped by laser radiation with a wavelength of 980 nm.
1. Experimental section. Synthesis of α-NaYF4/Yb+3/Er+3 and β-NaYF4/Yb+3/Er+3 - fluoride nanocrystals
For preparing fluoride nanocrystals with composition NaY0.78F4/Yb0.2/Er0.02 and cubic (α-phase) and hexagonal (β-phase) crystal lattices commercially-available reagents were used: oxides of yttrium, ytterbium and erbium; sodium carbonate; oleic acid 90% ; 1-octadecene 90% (Sigma-Aldrich) and trifluoroacetic acid 99% (PanReac). Sodium oxides and carbonate were used without pretreatment. Trifluoroacetic acid was purified by distillation and diluted with distilled water to 50% vol. Synthesis of nanophosphors was carried out by the method of thermal decomposition of trifluoroacetates of rare earth elements and sodium in oxygen-free medium and in a mixture of oleic acid and 1-octadecene [1, 2].
Preparation of trifluoroacetates
A stoichiometric mixture of yttrium oxides (176.3 mg or 0.78 mmol), ytterbium (78.8 mg or 0.2 mmol), erbium (7.6 mg or 0.02 mmol) and sodium carbonate (106.0 mg or 1 mmol) were dissolved in 15 ml of 50% vol. trifluoroacetic acid. The reaction mixture was stirred at a temperature of 72°C for 60 min. The resulting clear solution was evaporated via rotary evaporator at 100 °C and a residual pressure of 10 Torr until a free-flowing white powder was obtained, and then completely dried within 2 h. Scheme of reaction for obtaining trifluoroacetates of yttrium, ytterbium, erbium and sodium is shown in Fig. 1.
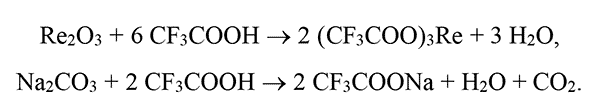
Figure 1. Scheme of reaction for obtaining trifluoroacetates of yttrium, ytterbium, erbium and sodium. Re = Y, Yb, Er.
Synthesis of α-NaYF4/Yb+3/Er+3 nanophosphors in a cubic α-phase
The resulting mixture of trifluoroacetates was transferred into a 100 ml four-necked flask equipped with a thermometer, an argon supply system and a vacuum valve, 30 ml of oleic acid, and into this flask 30 ml of 1-octadecene were added. With active stirring via magnetic stirrer, the flask was connected to a vacuum pump, and the temperature of reaction mixture was raised to 100°C by immersing the flask in Rose's alloy (in order to remove dissolved oxygen and water molecules from suspension). Pressure in the system was gradually reduced to 3 - 6 mbar, making sure that there was no violent foaming. After foaming ceased, the solution was held under vacuum for 30 min, and then the flask was purged with argon for 20 min.
Continuing the argon purge, the temperature of Rose's alloy was increased to 280°C at a rate of 20°C/min. Upon reaching a temperature of 240 - 250°C, trifluoroacetates began to decompose [1, 2], which manifested itself in the form of white smoke from the flask, and the solution itself became cloudy due to nucleus formation and growth of nanocrystals. A possible scheme of reaction for trifluoroacetates decomposition is shown in Fig. 2.
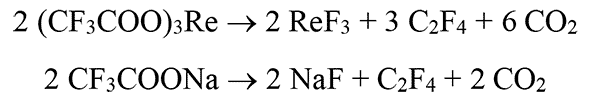
Figure 2. A possible scheme of reaction for trifluoroacetates decomposition of rare earth elements and sodium, followed by formation of nanophosphors α-NaYF4/Yb+3/Er+3. Re = Y, Yb, Er.
The solution was stirred at 280°C for 135 min, with monitoring the formation of fluoride nanocrystals by changing the integral intensity Iint_PL and IPL spectrum of fluorescence (PL) in upconversion within the spectral range of 350 - 900 nm. The photoluminescence of nanoparticles in solution was excited using a special fiber-optic probe under the action of a diode laser (with a wavelength of 980 nm). The probe contained a silica fiber for pumping and a receiving fiber, which captured the PL-signal from nanoparticles in the flask and directed it to FSD-10 mini-spectrometer (NTC Fiber Optic Devices, Russia). In Fig. 3a shows the dependences of Iint_PL on synthesis time t within the wavelength spectral ranges of 521±10 nm, 540±12 nm and 653±12 nm of photoluminescence, and in Fig. 3b shows the PL-spectra of IPL at different time intervals after the solution reached a temperature of 280°C. The characteristic PL-peaks in upconversion at wavelengths of 521 nm, 540 nm and 652 nm (see Fig.3b) are due to energy structure of rare-earth Er3+ ions in NaYF4 crystal, specifically, due to 2H11/2→4I15/2, 4S3/2→4I15/2 и 4F9/2→4I15/2 transitions (see Fig. 3 in [14]).
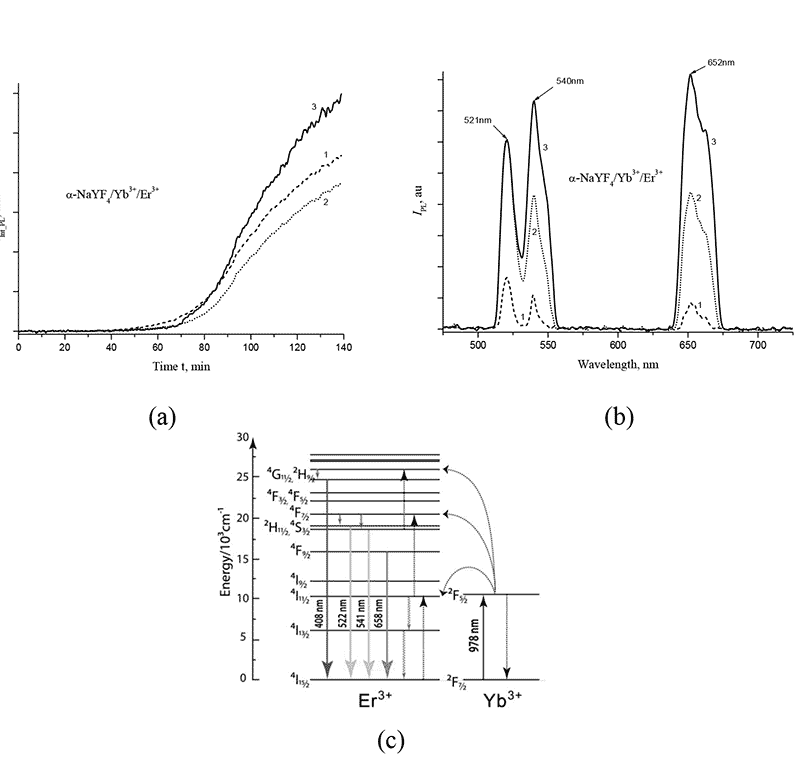
Figure 3. (a) Dependences of integrated PL-intensity Iint_PL for α-NaYF4/Yb+3/Er+3 nanophosphors on time t within the wavelength ranges 521±10 nm, 540±12 nm and 652±12 nm during synthesis at 280°C; (b) PL-spectrum of IPL nanoparticles after 60 min (1), 100 min (2) and 135 min (3) after reaching a temperature of 280°C; (c) simplified energy levels system for ytterbium and erbium. The arrows indicate emission transitions.
As seen from Fig. 3a, the intensity of photoluminescence in upconversion begins to increase sharply after the end of induction period, which lasts about 50 min at a synthesis temperature of 280 °C. By the 135th min, the growth rate of PL decreases, the photoluminescence intensity reaches its maximum and does not change further.
As a result of synthesis in the solution of oleic acid and 1-octadecene, α-NaYF4/Yb+3/Er+3 nanoparticles with an average size of 72 nm were formed, which was confirmed by measurements via X-ray diffractometer (see below). After formation of nanophosphors in α-phase, the resulting solution was used for synthesis of beta-nanophosphors.
Synthesis of β-NaYF4/Yb+3/Er+3 nanophosphors in the hexagonal β-phase
It should be noted that β-phase of NaYF4/Yb+3/Er+3 nanoparticles is formed, as a rule, under more “severe” synthesis conditions than α-phase. Therefore, to transform α-nanoparticles into β-NaYF4/Yb+3/Er+3 nanocrystals, a solution of α-nanoparticles in oleic acid and 1-octadecene was poured into a heat-resistant 48 ml glass flask (Chemglass, USA) withstanding a pressure of up to 12 atm. After blowing-off the solution with argon for 20 min the flask was hermetically sealed and immersed into Rose's alloy heated to 380°C. As the temperature in this flask increased to 380°C, the pressure in it increased to 10 atm, which was determined by pressure gauge built into the flask.
The process for preparing β-NaYF4/Yb+3/Er+3nanophosphors under the conditions described above was monitored by a change in intensity and PL-spectrum of suspension in upconversion. In Fig. 4 shows the time dependences of IInt_PL within the ranges 521±10 nm, 540±12 nm and 652±12 nm, as well as the IPL spectra after 40, 80, and 88 min the solution reaches a temperature of 380 °C. As seen from Fig. 4a, the integrated PL-intensity of suspension first decreases, which may testify to decrease in the size of nanoparticles due to their dissolution. After about 60 min the photoluminescence begins to increase, with the intensity of peak centered near 654 nm exceeding the intensity of the peak centered near 522 and 654 nm (see Fig. 4b).
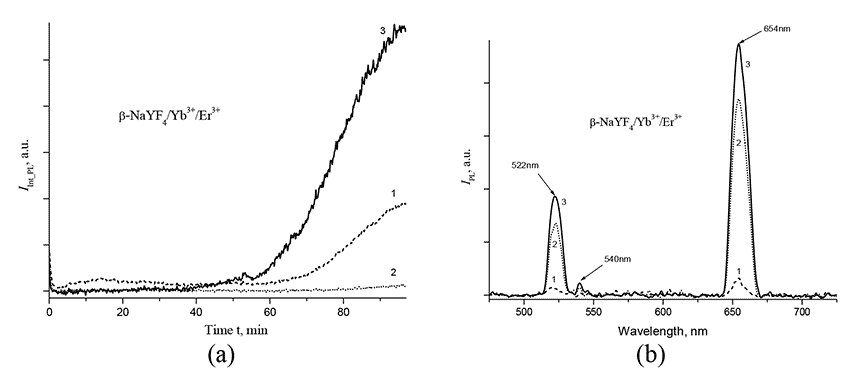
Figure 4. (a) Dependence of integrated photoluminescence intensity IInt_PL of of β-NaYF4/Yb+3/Er+3 nanophosphors within ranges of 521±10 nm, 540±12 nm и 652±12 nm on the time t during synthesis at 380°C and pressure of 10 atm; (b) - IPL PL spectrum of β-NaYF4/Yb+3/Er+3 nanoparticles after 40 min (1), 80 min (2) and 88 min (3) after the solution reaches a temperature of 380°C.
After 88 min the flask was removed from Rose's alloy and after its natural cooling to 40 °C the flask was opened, the solution was poured into plastic bottles with a volume of 50 ml (in 10 ml portions) and diluted to 50 ml with isopropanol. The precipitate was centrifuged for 15 min at 3000 rpm, after which the solution was decanted. All fractions were collected in one vial, added 10 ml of hexane, diluted with isopropanol to 50 ml and centrifuged again. The process of washing and centrifugation was repeated several times.
2. Study of crystal structure and size of nanophosphors
Crystal structure of synthesized nanophosphors was studied via Rigaku Miniflex600 X-ray diffractometer (Cu,λ = 1.54184 Å) within the range of incidence angles 2 = 15 - 70 deg. Diffractograms of nano-crystalline powders α-NaYF4/Yb+3/Er+3 и β-NaYF4/Yb+3/Er+3 are shown in Fig. 5a and Fig. 5b, respectively. It follows from analysis of these figures that the characteristic peaks observed at diffractograms refer to the pure α- and pure β-phases of NaYF4/Yb+3/Er+3 nanocrystals.
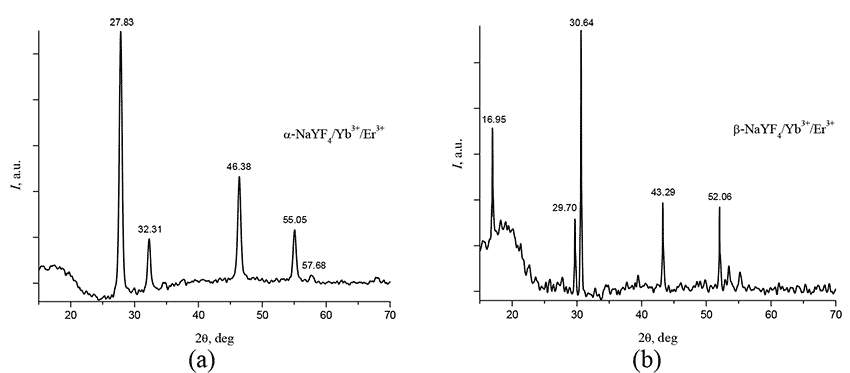
Figure 5. (a) Diffractograms of nano-crystalline powders α-NaYF4/Yb+3/Er+3, obtained via Rigaku Miniflex600 X-ray diffractometer (a) and β-NaYF4/Yb+3/Er+3. is the angle of incidence of X-ray beam relative the sample; (b) Diffractograms of nano-crystalline powders β-NaYF4/Yb+3/Er+3, obtained via Rigaku Miniflex600 X-ray diffractometer.θ -is the angle of incidence of X-ray beam relative the sample.
Note that the synthesis of β-NaYF4/Yb+3/Er+3 nanoparticles is usually carried out with an excess of sodium trifluoroacetate [1, 2]. In the case of synthesis at a pressure of 10 atm. As shown by our results, such an excess is not required.
The average diameter <D> of α-NaYF4/Yb+3/Er+3 nanophosphors and dispersion of their size were measured by dynamic light scattering in n-hexane via 90Plus_Zeta nanoparticle size analyzer (Brookhaven Instruments, USA). Hexane was chosen because the olein-coated NaYF4/Yb+3/Er+3 nanoparticles dissolve well in it, while the refractive index of n-hexane is nD = 1.375 at 20 °C, which is noticeably different from the refractive index of nanophosphors nD = 1.486 [15]. In Fig. 6 shows the distribution of synthesized α-NaYF4/Yb+3/Er+3 nanoparticles as a function of their diameter D. It can be seen that the average diameter of nanophosphors is <D> 72 nm.
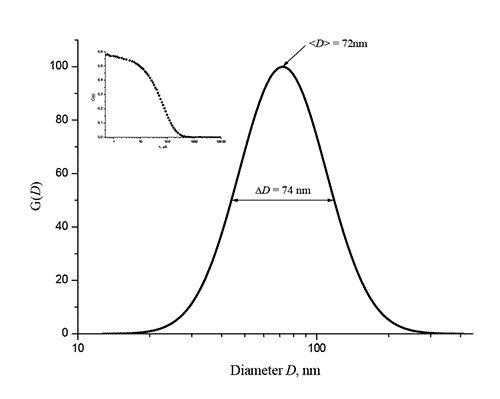
Figure 6. Distribution G (D) of synthesized α-NaYF4/Yb+3/Er+3 nanophosphors depending on the particle diameter D, measured by dynamic light scattering in n-hexane. The inset shows the corresponding autocorrelation function of scattered light intensity C (τ), where is delay time.
In Fig. 7 shows photographs of synthesized β-NaYF4/Yb+3/Er+3 nanophosphors obtained via Phenom ProX scanning electron microscope (Thermo Fisher Scientific). It is seen that nanophosphors have a hexagonal shape, which corresponds to crystalline β-phase. The sizes of nanophosphors, as follows from Fig. 7, are within the range 1 - 2 μm.
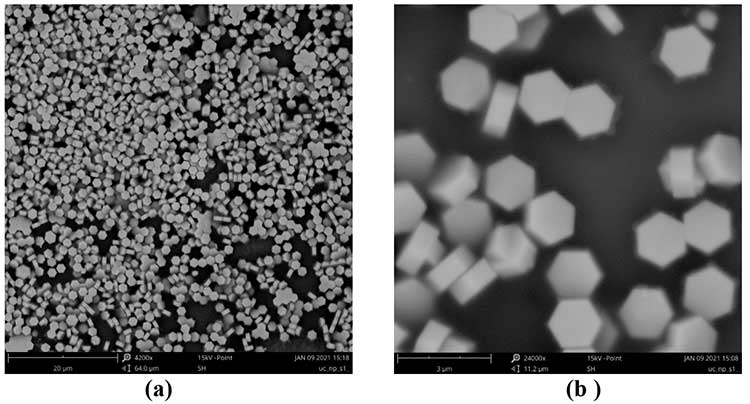
Figure 7. Photographs of synthesized β-NaYF4/Yb+3/Er+3 nanophosphors obtained via scanning electron microscope Phenom ProX at magnifications 4200х (a) и 24000х (b).
Results and discussion
Synthesis of nanophosphors NaYF4, NaLuF4, etc. with a predetermined crystal structure (α- and β-phases) is essential for medicine and technology. herewith, the formation of β-NaYF4/Yb+3/Er+3 nanoparticles is usually carried out under more severe conditions than the synthesis of α-NaYF4/Yb+3/Er+3 nanoparticles, specifically, at a higher temperature and with excess of sodium trifluoroacetate [1, 2]. It was shown that at a pressure of 10 atm and a temperature of 380°C, the synthesis of β-NaYF4/Yb+3/Er+3 particles is reliably realized at a stoichiometric ratio of reagents, i.e. without excess of sodium trifluoroacetate. This makes it possible to obtain more pure crystals, without the impurity of fluorides (NaF, etc). The synthesized nanophosphors can be used to create various active integrated optics devices, in particular, upconversion waveguide lasers and waveguide amplifiers for the telecommunication in C - wavelength range 1530 - 1565 nm [16].
Conclusion
Fluoride nanocrystals doped with rare earth elements can have a cubic or hexagonal crystal lattice (so-called α- and β-phases). By the example of β-NaYF4/Yb+3/Er+3 nanophosphors, it was shown that the transformation of α-nanoparticles into β-nanoparticles in a solution of oleic acid and 1-octadecene reliably occurs at a reactor pressure of 10 atm and a stoichiometric ratio for rare earth trifluoroacetates and sodium. The authors believe that that this conclusion is also valid for nanoparticles of NaLuF4, BaYF5, etc. types, doped with various rare-earth elements.
Acknowledgments
This work was financially supported by Ministry of Science and Higher Education of the Russian Federation within a framework of the state assignment of Federal Research Center "Crystallography and Photonics" of Russian Academy of Sciences as a part the study of photoluminescent properties of fluoride nanocrystals doped with rare-earth elements, and as a part of crystalline phase synthesis (within the grant no. 20-07-01038 of Russian Foundation for Basic Research). The authors are grateful to N.V.Minaev for help in studying nanophosphors by scanning electron microscopy.
References
- Mai H.-X., Zhang Y.-W., Si R., Yan Z.-G., Sun L.-D., You L.-P., Yan Ch.-H., High-Quality Sodium Rare-Earth Fluoride Nanocrystals: Controlled Synthesis and Optical Properties, J. Am. Chem. Soc, 2006, 128(19), 6426-6436.
- Mai H.-X., Zhang Y.-W., Sun L.-D., Yan C.-H., Size- and phase-controlled synthesis of monodisperse NaYF4:Yb,Er nanocrystals from a unique delayed nucleation pathway monitored with upconversion spectroscopy, J. Phys. Chem. C, 2007, 111, 13730-13739.
- Ye X., Collins J.E., Kang Y., Chen J., Chen D.T.N., Yodh A.G., Murray C.B., Morphologically controlled synthesis of colloidal upconversion nanophosphors and their shape-directed self-assembly, Proc. Nat. Acad. Sci. USA, 2010, 107(52), 22430-22435.
- Liu X., Zhang X., Tian G., Yin W., Yan L., Ruan L., Yang Z., Xiao D., Gu Z., A simple and efficient synthetic route for preparation of NaYF4 upconversion nanoparticles by thermo-decomposition of rare-earth oleates, Cryst. Eng. Comm., 2014, 16, 5650-5661.
- Alyatkin S., Asharchuk I., Khaydukov K., Nechaev A., Lebedev O., Vainer Y., Semchishen V., Khaydukov E., The influence of energy migration on luminescence kinetics parameters in upconversion nanoparticles, Nanotechnology, 2017, 28. 035401.
- Guryev E.L., Volodina N.O., Shilyagina N.Y., Gudkov S.V., Balalaeva I.V., Volovetskiy A.V., Lyubeshkin A.V., Sen A.V., Ermilov S.A., Vodeneev V.A., Petrov R.V., Zvyagin A.V., Alferov Z.I., Deyev S.M., Radioactive (90Y) upconversion nanoparticles conjugated with recombinant targeted toxin for synergistic nanotheranostics of cancer, Proc. Nat. Acad. Sci. USA, 2018, 115(39), 9690-9695.
- Rapaport A., Milliez J., Bass M., Cassanho A., Jenssen H., Review of the properties of up-conversion phosphors for new emissive displays, Journal of display technology, 2006, 2(1), 68-78.
- Hoeppe H.A., Recent developments in the field of inorganic phosphors, Angew. Chem., Int. Ed., 2009, 48, 3572-3582.
- Zhu H., Chen X., Jin L.M., Wang Q.J., Wang F., Yu S.F., Amplified spontaneous emission and lasing from lanthanide-doped up-conversion nanocrystals, ACSNano, 2013. 7(12), 11420-11426.
- Zhai X., Li J., Liu Sh., Liu X., Zhao D., Wang F., Zhang D., Qin G., Qin W. Enhancement of 1.53 μm emission band in NaYF4:Er3+,Yb3+,Ce3+ nanocrystals for polymer-based optical waveguide amplifiers, Optical Materials Express, 2013, 3(2), 270-277.
- Wang Y., Guo X., Liu Sh., Zheng K., Qin G., Qin W., Controllable synthesis of -NaLuF4:Yb3+,Er3+ nanocrystals and their application in polymer-based optical waveguide amplifiers, Journal of Fluorine Chemistry, 2015, 175, 125-128.
- Chen G.F.R., Zhao X., Sun Y., He Ch., Tan M.Ch., Tan D.T.H., Low loss nanostructured polymers for chip-scale waveguide amplifiers, Scientific Reports, 2017, 7, 3366.
- Sokolov V.I., Asharchuk I.M., Molchanova S.I., Nazarov M.M., Nechaev A.V., Khaydukov K.V., Optical amplifier for C-band based on polymer waveguide with embedded nanophosphors doped with rare earth elements, Materials of the IV International Scientific Conference "Problems of Interaction of Radiation with Matter", Gomel, 2016, 2(127), 132 (in Russian).
- Znamenskiy N.V., Malyukin Yu.V., Spectra and dynamics of optical transitions of rare-earth ions in crystals, M: Fizmatlit, 2008, 191 p. (in Russian).
- Sokolov V.I., Zvyagin A.V., Igumnov S.M., Molchanova S.I., Nazarov M.M., Nechaev A.V., Savelyev A.G., Tyutyunov A.A., Khaydukov E.V., Panchenko V.Y., Determination of the refractive index of β-NaYF4/Yb3+/Er3+/Tm3+ nanocrystals using spectroscopic refractometry, Optics and Spectroscopy, 2015, 118(4), 609-613 (in Russian).
- Sokolov V.I., Akhmanov A.S., Asharchuk I.M., Igumnov S.M., Molchanova S.I., Nechaev A.V., Savelyev A.G., Tyutyunov A.A., Khaydukov E.V., Khaydukov K.V., Panchenko V.Y. Integrated optics based on nanocomposite polymer materials, Vestnik RFFI, 2015, 4(88). 68-79 (in Russian).
ARTICLE INFO
Received 15 January 2021
Accepted 19 January 2021
Available online February 2021
Recommended for publication by PhD V. Don
Fluorine Notes, 2021, 134, 1-2
