Received: December 2020
DOI 10.17677/fn20714807.2020.06.04
Fluorine Notes, 2020, 133, 7-8
PREPARATION OF HEXAFLUOROACETONE BY OXIDATION OF 2,2,4,4-TETRAKIS(TRIFLUOROMETHYL)TIETHANE WITH SODIUM NITRITE
V.E. Boyko, M.D. Molchanov, V.L. Don, S. M. Igumnov
A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Vavilov St. 28, Moscow, 119991, Russian Federation
e-mail: boykii@mail.ru
NPO PIM-INVEST LLC, Leninsky Prospect, 47, Moscow, 119991, Russian Federation
Abstract: A convenient method for producing hexafluoroacetone from perfluoropropene
by oxidation of 2,2,4,4-tetrakis(trifluoromethyl)tiethane with sodium nitrite is proposed.
Keywords: hexafluoroacetone, 2,2,4,4-tetrakis(trifluoromethyl)tiethane, sodium nitrite
Hexafluoroacetone is one of the most important products of organofluorine chemistry, it is widely used in organic synthesis, since trifluoromethyl groups, having a significant effect on neighboring groups in the molecule, are able to modify the physical properties of a substance in desired way [1]. It is an important raw material for the production of fluorinated monomers, in particular acrylates, used in photolithography [2]. Hexafluoroacetone is used in peptide synthesis to protect amino groups with simultaneous selective activation of the alpha-carboxyl group in amino acids containing two carboxyl groups (aspartic, malic) by the formation of five-membered lactones [3]. The reduction of hexafluoroacetone gives hexafluoroisopropanol –a highly demanded solvent in the modern electronic industry and the key raw material for the production of various pharmaceutical products, in particular latest generation anesthetic, sevoflurane [4].
Hexafluoroacetone (HFA) is obtained by the following methods: fluorination of hexachloroacetone with hydrogen fluoride in gas phase at high temperatures [5], oxidation of hexafluoropropene in a gas phase with oxygen on a catalyst [6], isomerization of perfluoropropene oxide under action of Lewis acids – antimony pentafluoride [7] or aluminum chlorofluoride [ 8 ] and oxidation of 2,2,4,4-tetrakis (trifluoromethyl) tiethane with aqueous solution of sodium iodate with yield 64 -69% [9].
Looking for a simple method for preparing HFA that does not require the use of complex equipment, we have chosen the oxidation of 2,2,4,4-tetrakis (trifluoromethyl) thiethane 1. Compound 1 was obtained by the reaction of perfluoropropene with elemental sulfur in dimethylformamide with potassium fluoride according to the described procedure with a yield of more than 90%in our hands [9].
The preparation of HFA by oxidation 1 with an aqueous solution of sodium iodate with a yield of 64-69% is described [9], however, sodium iodate is an expensive and uncommon reagent.A number of other processes for the production of HFA by oxidation of 1 are discribed, which, despite the good yields, require complicated equipment for gas phase operation at high temperatures [10], under UV radiation [11], work with oxygen [12].
We chose sodium nitrite as an oxidizing agent, which, unlike sodium iodate, is available and cheap. Nitrogen (III) of nitrite ion is reducing to nitrogen (II) of NO. Oxidation was carried out in aqueous acetonitrile; we also tried other solventsfor this purpose –benzonitrile, methanol, isopropanol, dimethylformamide.
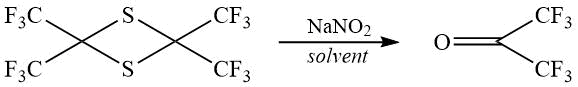
The maximum yield in oxidation of 1 with sodium nitrite was 58%, despite the fact that in some cases, according to the 19FNMR data, complete conversion of the starting material was achieved, and we did not observe any by-products.Therefore we concluded that gaseous productswhich boil below -77 ° C and do not condense into a trap with a mixture of dry ice and acetone are formed as by-products.Our experiments have shown that an increase in the amount of nitrite from 2.1 to 5.0 moles per mole 1 does not lead to an increase in the yield. Our attempts to increase the yield by adding a second oxidant, isopropyl nitrite, tert-butyl hydroperoxide, or nitric acid to the reaction mixture also failed.Other oxidants that we tried to use, namely sodium hypochlorite and calcium oxide, caused the formation of some thio derivatives as byproducts, and thus to a decrease in the yield of HFA.
The experimental results are summarized in Table 1.
Table 1. Results of the reaction of 2,2,4,4-tetrakis (trifluoromethyl) thiethane 1 with oxidizing agents depending on the ratio of reagents in various solvents.
|
Entry |
1 : oxidant (mol ratio) |
Solvent : water (volume ratio) |
Conversion / yield |
|
1 |
1 : NaNO2 ( 1 : 2,1 ) |
CH3CN : H2O ( 2 : 1 ) |
100 / 58 |
|
2 |
1 : NaNO2 ( 1 : 2,1 ) |
CH3CN |
100 / 58 |
|
3 |
1 : NaNO2 : HNO3 ( 1 : 2,1 : 0,5 ) |
CH3CN : H2O ( 2 : 1 ) |
32 / 16 |
|
4 |
1 : NaNO2 ( 1 : 3 ) |
CH3CN |
100 / 58 |
|
5 |
1 : NaNO2 ( 1 : 5 ) |
CH3CN |
100 / 58 |
|
6 |
1 : CaO ( 1 : 2,1 ) |
i-PrOH |
51 / 16 |
|
7 |
1 : NaNO2 : t-BuOOH ( 1 : 2,1 : 0,5 ) |
CH3CN : H2O ( 2 : 1 ) |
27 / 14 |
|
8 |
1 : NaNO2 ( 1 : 2,1 ) |
C6H5CN : H2O ( 2 : 1 ) |
95 / 58 |
|
9 |
1 : NaNO2 ( 1 : 2,1 ) |
СH3OH : H2O ( 10 : 1 ) |
100 / 58 |
|
10 |
1 : NaNO2 ( 1 : 2,1 ) |
СH3OH : H2O ( 5,5 : 1 ) |
100 / 58 |
|
11 |
1 : NaNO2 ( 1 : 2,1 ) |
СH3OH : H2O ( 4 : 1 ) |
100 / 58 |
|
12 |
1 : NaNO2 : i-PrONO ( 1 : 2,1 : 2,1 ) |
CH3CN : H2O ( 2 : 1 ) |
100 / 58 |
|
13 |
1 : NaNO2 : i-PrONO ( 1 : 2,1 : 2,1 ) |
i-PrOH : H2O ( 2 : 1 ) |
80 / 40 |
|
14 |
1 : NaNO2 : i-PrONO ( 1 : 3 : 2,1 )* |
C6H5NO2: H2O ( 1 : 1 ) |
100 / 50 |
|
15 |
1 : NaClO ( 1 : 2,1 ) |
DMF |
12.5 / 0 |
*Catalytic amount of triethyl benzyl ammonium chloride was added.
Despite the not very impressive yield (58%) the advantages of our method are simplicity and low cost and availability of reagents.
Experimental
General procedure, entry 1 (Table 1)
In a flask, equipped with mechanical stirrer thermometer dropping funnel and reflux condenser, connected with dry-ice condenser sodium nitrite (21 g, 0,3 mol) and water (45 ml) were added to acetonitrile (100 ml). Then reaction mixture was heated to 70 – 75ºC and 2,2,4,4-tetrakis(trifluoromethyl)tiethane (50 g, 0,137 mol) was added dropwise. Then the reaction mixture was refluxed for 2 hours. According to 19F NMR only HFA hydrates were presented in the reaction mixture. All the liquid contents were distilled from the reaction mass in a vacuum to dryness and then the distillate was distilled, separating acetonitrile and HFA hydrate, which was redistilled from sulfuric acid in a trap of 25 g HFA, 58% yield.
Acknowledgments
This work was performed with the financial support from Ministry of Science and higher Education of the Russian Federation using the equipment of Center for molecular composition studies of INEOS RAS.
References
- Amara J.P., Swager T.M., Macromolecules 2006, 39, 5753-9.
- Swinson J. PharmaChem 2012, 11(5/6), 16-20.
- Albericio F.; Burger K.; Cupido T.; Ruiz J.; Spengler J., ARKIVOC, Commemorative Issue in Honor of Prof. Eusebio Juaristi on the occasion of his 55th anniversary, 2005, 6, 191-199.
- Ramig K., Synthesis, 2002, 17, 2627-2631.
- CN 104710296 (2015).
- Kurosaki Akito, Okazaki Susumu ChemistryLetters, 1988, 1, 17-20.
- US 3213134 (1965).
- Petrov V. A. et al, Journal of Fluorine Chemistry, 1996, 77(2), 139-142.
- Van Der Puy, Michael and Anello, Louis G., Organic Syntheses, 1985, 63, 154-9.
- Middleton W. J., Sharkey W. H. J.. Org. Chem., 1965, 30(5), 1384-1390.
- CN 102976908 (2013).
- US 4337361 (1982).
Received 17 December 2020
Accepted 21 December 2020
Available online December 2020
Recommended for publication by PhD M. A. Manaenkova
Fluorine Notes, 2020, 133, 7-8
