Received: November 2020
DOI 10.17677/fn20714807.2020.06.03
Fluorine Notes, 2020, 133, 5-6
Surface properties of thin films of poly(2,3,4,5,6-pentafluorostyrene) and diblock copolymers based on 2,3,4,5,6-pentafluorostyrene and 2-hydroxyethyl methacrylate
K.E.Chekurov, A.I. Barabanova, I.V. Blagodatskikh, A.R. Khokhlov
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991, GSP-1, Moscow, B-334, Vavilov St. 28
e-mail: chekurov@polly.phys.msu.ru
Abstract: The surface properties of thin films obtained by applying poly(2,3,4,5,6-pentafluorostyrine) (PPFS) and amphiphilicdiblock copolymers (DC) of 2,3,4,5,6-pentafluorostyrene (PFS) and 2-hydroxyethylmethacrylate (HEMA) on smooth substrates (silicone plates) from solutions in THF and DMF, respectively, in the presence and absence of a cross-linking agent– hexamethylenediisocyanate (HMDI) were studied. Comparative testing of the DC films obtained in the presence and in the absence of HMDI showed that the DC films have the higher water and diiodomethane(DI) contact angles: θН2О = 108 ± 2º and θCH2I2 = 83 ± 1º, than a film of PPFS (θН2О = 98 ± 1º and θCH2I2 = 74 ± 1º).The energy characteristics of the thin films surfacewere determined by the results of measuring the water and DI contact angles. It was established that DC films have a lower specific free surface energy than films based on PPFS.
Keywords: 2,3,4,5,6-pentafluorostyrene, amphiphilicdiblock copolymers, surface properties.
Introduction
For creating omniphobic coatings the compounds containing long-chain fluorinated alkyl fragments with terminal –CF3 groups, capable of self-assembly with close packing of –CF3 groups, are usually used. The dense packing provides low values of the specific free surface energy of coatings ( = 6 -7 mJ/m2) [1] and makes them resistant to wetting by both polar and non-polar liquids. However, long fluorinated fragments do not decompose in nature, and the destruction of coatings is accompanied by the release of toxic perfluorooctane sulfonic and perfluorooctanoic acids [2]. In this regard, an urgent task is to create omniphobic coatings without the use of compounds with long-chain fluorinated alkyl fragments.
The solution of this problem is the coating of amphiphilic fluorine-containing block copolymers [3]–[9]. As a result of microphase separation, amphiphilic block copolymers create a nanoscaled rough surface with a low surface energy due to a high content of fluorine atoms on the surface, which leads to the realization of the Cassie state and an improvement in the repellent properties of coatings [3]–[9].
Previously, our group proposed to use amphiphilic diblock copolymers (DC), consisting of poly(2,3,4,5,6-pentafluorostyrene) (PPFS) and poly(2-hexaethyl methacrylate) blocks, to create omniphobic coatings on cotton-polyester fabrics. The application to the fabric of the DC consisting of 32 HEMA-units and 197 PFS-units (H32-F197), creates a nanoscaled rough surface with a rather high content of fluorine atoms on the surface ([F] = 19.7%), which has superhydrophobic and oleophobic properties with water and diiodomethane (DI) contact angles (CA) θН2О = 158 4 ° and θCH2I2 = 107 3 ° (> 90 ° for PPFS), and hysteresis CA (CAH) = 5 2 ° [3]–[5].
This work continues these investigations, and its purpose is to comparatively study the surface properties and energy characteristics of thin films made of PPFS and H32-F197, copolymerapplied from organic solvents (THF and DMF) in the presence and absence of a crosslinking agent - hexamethylenediisocyanate (HMDI) on smooth silicon wafers.
Experimental part
Monomers 2,3,4,5,6-pentafluorostyrene (PFS) (98%, PIM-INVEST, Russia) and 2-hydroxyethyl methacrylate (HEMA) (98%, ZL Chemical, China) were distilled under vacuum before use (P = 0.1 mbar, T = 63° C and 105 °C for PFS and HEMA, respectively). The polymerization initiator α-bis-isobutyric acid dinitrile (AIBN) (98%, Sigma-Aldrich, Germany) was twice recrystallized from methanol. Chain transfer agent (or RAFT-agent) 2-cyano-2-propyl-dithiobenzoate (CPDB) (>97%, Sigma-Aldrich, Germany) and crosslinker hexamethylenediisocyanate (HMDI) (> 99%, Sigma-Aldrich, Germany) were used as received.The solvents were purified according to conventional techniques.
PPFS was synthesized by polymerization of PFS via reversible addition-fragmentation chain-transfer polymerization (RAFT polymerization) according to the procedure described in [3]–[5]. Polymerization of PPFS at [PFS] = 2 mol/l in N,N-dimethylformamide (DMF) at 60°C at a molar ratio of [CPDB]/[AIBN]=1.9 and an initiator concentration [AIBN] = 2.7 × 10-3mol/l. The resulting PPFS was precipitated in excess of methanol, followed by centrifugation for 10 min at 11000 rpm (Rotina R38, Germany). The polymers obtained were dried at room temperature under vacuum up to constant weight.
DCs were synthesized by two-stage RAFT polymerization. At the first stage, the PHEMA-RAFT-agent was obtained by polymerization of HEMA ([HEMA] = 2 mol / l) at [CPDB] = 2 × 10-2 mol / l and [AIBN] = 8 × 10-3 mol / l in DMF at 60 ° C. The PHEMA-RAFT-agent was precipitated in excess of chloroform, followed by centrifugation for 10 min at 11000 rpm (Rotina R38, Germany). At the second stage, DCs were synthesized by polymerization of PFS ([PFS] = 2 mol/ L) in the presence of PHEMA-RAFT-agent at [PHEMA] / [AIBN] = 5 in DMF at 60 ° C. At the end of the reaction, the product was precipitated in a 10-15 fold excess of chloroform, then centrifuged for 10 min at 11000 rpm (Rotina R38, Germany) and dried in a vacuum to constant weight. Conversion was determined gravimetrically. The composition was determined by elemental analysis (Table 1).
Molar masses (Mn and Mw) and polydispersity indexes (Mw/Mn) of PPFS, PHEMA and DCs (Table 1) were determined by gel permeation chromatography (GPC) using Agilent 1200 liquid chromatograph equipped with a refractometric detector and data processing system ChemStation 1200 (Agilent). PLmixC (Agilent) column was used for analysis of PPFS a temperature of 30°Сand eluent flow rate of 1 ml / min. G-gel column (spherical macroporous sorbent based on a hydrolyzed glycidyl methacrylate copolymer with ethylene dimethacrylate [10], [11]) was used at a temperature of 30°С and eluent flow rate of 0.5 ml / min. 0.03 M LiBr in DMF was used as an eluent for PHEMA; a mixture of THF : 0.03 M LiBr in DMF (50:50 vol.%) was used for analysis of DCs and for comparison with the initial PHEMA-RAFT-agent. The instrument was calibrated according to Waters and Merck polystyrene (PS) standards.
Table 1. Molecular weight characteristics of PPFS, PHEMA and DC
|
Sample |
Composition, mol. % |
Mn 10-3 |
Mw/Mn |
|
|
PFS |
HEMA |
|||
|
PPFS |
100 |
0 |
29.3 |
1.17 |
|
H321) |
0 |
100 |
6.6 |
1.23 |
|
H32-F1972) |
86 |
14 |
40 |
1.05 |
1) The letter “H” designates HEMA units. The subscripts associated with the H letter correspond to the degree of polymerization of PHEMA PnHEMA (or n) which was defined as Mn/130, where Mn – is the number average molecular weight of PHEMA as determined by GPC and 130 is the molar mass of HEMA; 2)The letters “H” and “F” designate HEMA and PFS units, respectively. The subscripts associated with the letters correspond to the degree of polymerization Pn of each polymer blocks. The degree of polymerization (PnPFS) of PPFS was calculated taking into account the content of PFS units in copolymer as follows: PnPFS = PnPHEMA × mPFS / mHEMA, where mPFS and mHEMA are the content of PFS and HEMA units in DC according to element analysis data.
The films were deposited onto silicon wafers from 50 mg/ml solution of PPFS in THF and DC in DMF in the presence and absence of HMDI by spin-coating in the operating mode for 15 sec at 1000 rpm and 60 sec at 3000 rpm. Before films deposition, silicon wafers were treated by boiling in 30% H2O2 solution (T = 107°С) for 50 min to form silanol SiOH groups on their surface [12]. The prepared samples were dried in vacuum (P = 6 × 10-2Pa) at 40°C for 24 h.
The hydrophobic and oleophobic properties of polymer films on the surface of silicon wafers were determined from the static water (θН2О) and DI (θCH2I2) CAs by the sessile drop method (volumes were 1.5 µL) using Kruss DSA 25 (Germany) device with the accuracy of 1°. The average of six readings was used as the data.
The chemical formulas of the compounds used are shown below:
|
Monomers |
HEMA |
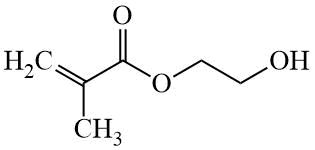 |
|
PFS |
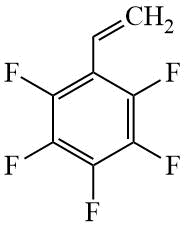 |
|
|
RAFT-agent |
CPDB |
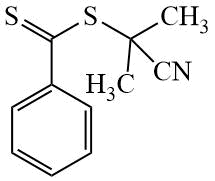 |
|
Initiator |
AIBN |
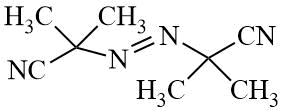 |
|
Crosslinker |
HMDI |
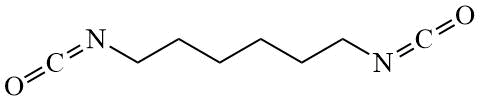 |
Chemical structures of the monomers, RAFT-agent, initiator and crosslinker for the preparation of thin films
Results and discussion
The wetting method was used to determined the energy characteristics of the surface of PPFS and DCs films deposited in the presence and absence of HMDI [13]. The values of the specific free surface energy (γSV), polar (γpSV) and dispersion (γdSV) components were calculated using the Owens-Wendt equation [13]:
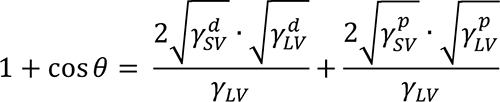
where θ– water and DI CAs, γpLV and γdLV– polar and dispersion components of liquid surface tension γLV. The accuracy of determining the γSV values is 1 mJ/m2. The values of polar and dispersive components of surface tension for test liquids (water and DI) are shown in Table 2.
Water and DI CAs θН2О and DI θCH2I2 on the surface of the films were determined by the sessile drop method. Drops of water and DI spread on the hydrophilic surface of the treated silicon wafers due to complete wetting. After applying PPFS and DCs, the plates become hydrophobic (Fig.1), and the value of θН2О of film coated with DC in presence of HMDI, is 108 2 º, which is 10 º higher than θН2О for a thin film of PPFS (θН2О = 98 1 º), despite the fact that the DC contains only 86 mol.% of hydrophobic PFS units. The static CA versus DI (droplet volume 1.5 µL) for PPFS and DC films are 74 1 º and 83 1 º, respectively (Table 2). Similar CA values are also observed for films from DC prepared without crosslinker HMDI: θН2О = 106 1º and θCH2I2 = 82 1º.
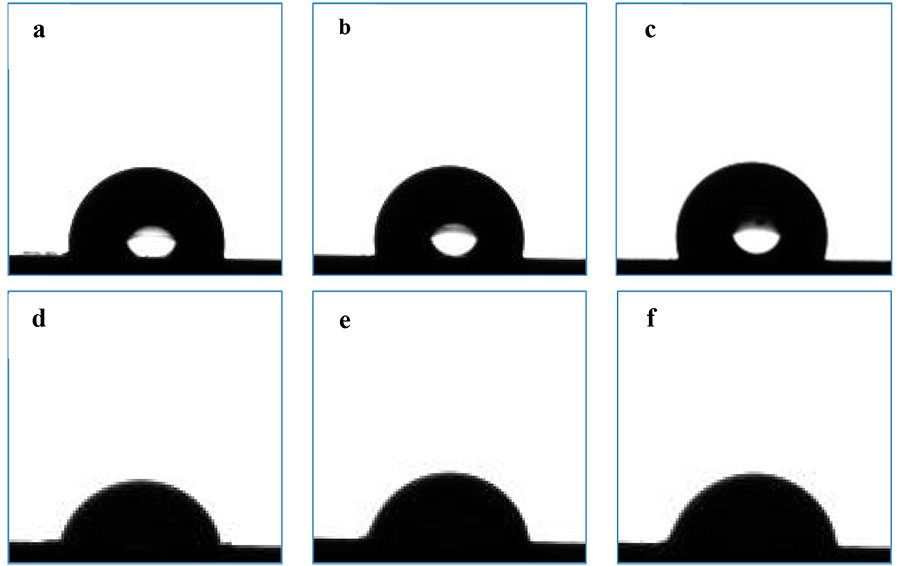
Figure 1. Instantaneous images of water and DI droplets (V = 1.5 µL) on the surface of PPFS (a, d) andH32-F197 films without HMDI (b, e) and with HMDI (c, f) on silicon wafers.
The observed improvement in the repellant properties of films from DC in comparison with the corresponding parameters for PPFS films can be associated with higher adhesion of the H32-F197 to the substrate surface [3]–[5]. In PPFS, there are no functional groups that are reactive with respect to SiOH groups on the surface of silicon wafers, which leads to low polymer adhesion and, as a consequence, to the formation of defective film. Due to the presence of OH-groups in the PHEMA blocks, DC binds to the substrate due to formation of weak hydrogen (in the absence of HMDI) or strong covalent (in presence of HMDI) bonds, and this leads to the formation of homogeneous defect-free coatings.
The values of the polar and dispersion components of the surface tension for water and DI, as well as the free surface energy, calculated from the data of the CA measurements for water and DI θН2Оand θCH2I2, are given in Table 2.
Table 2.Water and DI CAs and the values of the surface energy, as well as the dispersion and polar components of the surface energy of the PPFS and DC films, and test liquids, used in calculatingthe free surface energy of thin polymer films.
|
Sample |
θH2O |
θCH2I2 |
, mJ/m2 |
,mJ/m2 |
, mJ/m2 |
|
PPFS |
98 |
74 |
2.4 |
18.6 |
21.0 |
|
H32-F197 |
106 |
82 |
1.3 |
15.3 |
16.6 |
|
H32-F197 + HMDI |
108 |
83 |
1.0 |
15.0 |
16.0 |
|
Water |
- |
- |
50.8 |
21.8 |
72.6 |
|
DI |
- |
- |
2.3 |
48.5 |
50.8 |
Table 2 shows that on the surface of films based on PPFS and H32-F197, prepared both in the presence and in the absence of HMDI, fluorinated fragments are predominantly concentrated. Indeed, the calculated values of the free surface energy below = 25.6 mJ/m2, characteristic of polytetrafluoroethylene (PTFE) [14] and above = 6 - 7 mJ/m2, typical for close packing of –CF3-groups [1]. It is important to note that the presence of hydrophilic PHEMA block in the composition of the DC did not lead to an increase in the surface energy, but, on the contrary, reduced its values in comparison with the values obtained for the PPFS films. This is probably due to the microphase separation of DCs in the films and the formation of nanosized domains with a predominant content of one of the polymer blocks. The investigation of the H32-F197 coating on smooth surface using transmission electron microscopy showed that it is characterized by a spherical morphology with a sphere diameter of 30 nm [5].
The films based on H32-F197 prepared with HMDI have lower values of both the total surface energy and its dispersion and polar components than films from PPFS and DC prepared without HMDI. This may be due to the fact that a DC covalently bonded to a smooth surface, completely covers it, thereby creating a more energetically homogeneous surface.
Conclusion
Thus, the introduction of hydrophilic PHEMA block into PPFS provides uniform and homogeneous deposition of the copolymer on the hydrophilic surface of silicon wafers, reduces the specific free surface energy of the resulting coating, and improves its repellent properties with respect to water and DI.
Acknowledgments
The work was financially supported by Russian Science Foundation (project № 17-13-01359-П) in A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences (INEOS RAS).
Elemental analysis were performed with the financial support from Ministry of Science and Higher Education of the Russian Federation using the equipment of Center for molecular composition studies of A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences.
References
- Nishino T., Meguro M., Nakamae K., Matsushita M., Ueda Y., Langmuir, 1999, 15, 4321‑4323.
- Buck, R. C., Franklin, J., Berger, U., Conder, J. M., Cousins, I. T., de Voogt P.,Jensen A.A., Kannan K., Mabury S.A., van Leeuwen S. PJ., Integr. Environ. Assess. Manag., 2011, 7, 513-541.
- Chekurov K.E., Barabanova A.I., Blagodatskikh I.V., Lokshin B.V., Kondratenko M.S., Gallyamov M.O., Peregudov A.S., Khokhlov A.R., Appl. Polym. Sci., 2021, V. 138, 49714.
- Chekurov K.E., Barabanova A.I., Blagodatskikh I.V., Peregudov A.S., Khokhlov A.R., Fluorine notes, 2019, 2(123), 1-2.
- Chekurov K.E., Barabanova A.I., Blagodatskikh I.V., Lokshin B.V., Peregudov A.S., Abramchuk S.S., Khokhlov A.R., Dokl. Chem., 2019, 484(2), 33-36.
- Zou H., Lin S., Tu Y., Liu G., Hu J., Li F., Miao L., Zhang G., Luo H., Liu F., Hou C., Hu M., J. Mater. Chem. A., 2013, 1, 11246-11260.
- Shi Z., Wyman I., Liu G., Hu H., Zou H., Hu J., Polymer, 2013, 54, 6406-6414.
- Li Y., Zheng X., Xia Z., Lu M., Prog. Org. Coatings, 2016, 97, 122-132.
- Li G., Zheng H., Wang Y., Wang H., Dong Q., Bai R., Polymer, 2010, 51(9), 1940-1946.
- Tennikova T.B., Horak D., Svec F., Kolar J., Coupek J., Trushin A., Maltzev V.G., Belenkii B.G., J. Chromatogr., 1988, 435, 357-362.
- Fomenkov A. I., Blagodatskikh I. V., Ponomarev I. I., Volkova Y. A., Ponomarev I. I., Khokhlov A. R., Polym. Sci. Ser. B, 2009, 51(5), 166-173.
- Rama Mohana Rao B.V., Basu P.K., Biswas J.C., Lahiri S.K., Solid State Commun., 1996, 97(5), 417-418.
- Kloubek J., Adv. Colloid Interface Sci., 1992, 38, 99.
- Dettre R. H. and Johnson R. E., J. Colloid Interface Sci., 1969, 31, 568.
ARTICLE INFO
Received 04 December 2020
Accepted 06 December 2020
Available online December 2020
Recommended for publication by PhD A.A. Tyutyunov.
Fluorine Notes, 2020, 133, 5-6
