Received: November 2020
DOI 10.17677/fn20714807.2020.06.01
Fluorine Notes, 2020, 133, 1-2
STRUCTURE OF PARAMAGNETIC CENTERS, GENERATED FROM POLYFLUORINATED ALCOHOLS
S. V. Kudashev1, V. A. Babkin2, D. S. Andreev2, A. V. Ignatov2, N. V. Kuznetsova1, M. D. Kosogorina1
1Volgograd State Technical University (Architecture and Construction Institute), 400005 Volgograd, Lenin ave., 28, e-mail: kudashev-sv@yandex.ru
2Volgograd State Technical University (Sebryakovsky br.), 403343 Volgograd Region, Mikhailovka, Michurina st., 21, e-mail: babkin_v.a@mail.ru
Abstract:. The structure of paramagnetic centers formed from polyfluorinated alcohols was investigated by quantum-chemical semi-empirical MNDO- and DFT (6–31G)-methods. The calculation of g-factor value and electronic-geometric structure of resulting free-radical particles was performed and conformations of radical particles characterized by the smallest total energy value were determined. Based on the presented results of quantum chemical calculations and EPR, it was shown that the total energy (E0) of ●(CF2CF2)nCH2ОН radical is 128 kJ mol-1 higher than the E0 for Н(CF2CF2)nCH2О● Н(CF2CF2)nCH2О● radical, and their structures are linear.
Keywords: polyfluorinated alcohols, fluorinated free radicals, fluoropolymers, quantum-chemical calculation, MNDO method, DFT method, paramagnetic centers, EPR.
Introduction
Fluorine-containing polymers attract the interest of researchers because of unique properties of materials obtained [1–4]. Introduction of fluorinated (including poly- and perfluorinated) compounds (i.e. fluoromonomers, fluorinated alkanes and ethers, organofluorine peroxides, fluoroalcohols, polyfluoroalkyl(meth)acrylates and polyfluoroalkyl fumarates, polyfluoroalkylchlorosulfites, fluorinated ε-caprolactam acid and polytetrafylene polymethylene and polytetrafylene fluoroplastics, fluoroelastomers) can be carried out both at the stage of manufacturing of polymers, and also at the stage of their processing (by surface modification of finished products [5–9]).
A special place among the aforementioned modifying additives is occupied by polyfluorinated alcohols-telomers H(CF2CF2)nCH2OH (n = 1–6), manufactured in industry by interaction of tetrafluoroethylene with methanol (which in small amounts provide an improvement in the properties of a number of heterochain polymers), making it possible to obtain cord-, textile-, medical-purposes and rubber-technical fluorine-containing materials with controlled hydrophobicity, as well as materials with special properties [10–13]. The most pronounced positive effect on properties of modified polymers was produced by compositions based on polyfluorinated alcohol with a telomerization degree n = 4 [11–13].
The unique chemical structure of polyfluorinated alcohols containing in a molecule simultaneously two proton-donor HCF2–, НО– and also – the proton-acceptor –CF2–CF2– groups (in contrast to F(CF2CF2)nCH2OH) alcohols) determines the possibility of their chemical and physicochemical interaction with polymer macromolecules, promoting the reorganization of their supramolecular structure [11, 14]. In addition, the possible generation of electrophilic free radicals from polyfluorinated alcohols ●(CF2CF2)nCH2OH, which leads to termination of radical processes (both in the gaseous and condensed phases) and act to raise in thermal-, fire- and light resistance of fluoropolymer composites [12]. In this regard, the aim of this report is to study the structure of paramagnetic centers generated from polyfluorinated alcohols using quantum chemistry methods and determination of EPR spectral characteristics.
Results and Discussion
The review papers [15] are devoted to the study and identification of organic fluorinated free-radical particles. Thus, the most studied particles are fluorinated radicals with normal and branched structure in irradiated perfluoroalkanes and polytetrafluoroethylene [16].
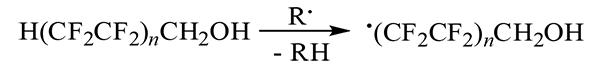
The generation of free terminal radicals from polyfluorinated alcohols can occur as a result of interaction (for example, with macroradicals in polymers modified by these compounds) under the conditions of their thermal- or radiation-exposure and light aging:
Geometric and electronic structure of fluorinated free radicals ●(CF2CF2)nCH2OH (I) (see Table 1) and Н(CF2CF2)nCH2О● (II) (with a charge of total radical system = 0 and multiplicity = 2) (see Tables 1–4 and Figures 1–2) was investigated by semi-empirical quantum chemical MNDO method (built in Firefly program), which is partially based on the source code GAMESS (US) [17, 18, 20], in the approximation of isolated particle in gaseous phase and with geometry optimization in all parameters using the standard gradient method.
The structures of studied radicals optimized in all parameters were obtained. As can be seen, an increase in a number of –CF2–CF2– groups in radical structure provides a natural increase in total energy E0. Herewith, the dipole moment for radical (I) increases (although insignificantly) with increase in a number of groups, while for radical (II) its dipole moment decreases (see Tables 1 –3).
Table 1. Optimized bond lengths, bond angles and atomic charges for ●CF2CF2CH2OH1 particle
|
Bond lengths |
R, Å |
Bond angles |
Angles |
Atoms |
Atomic charges |
|
C(2)–O(1) |
1,39 |
C(2)–O(1)–H(5) |
112 |
O(1) |
–0,308 |
|
C(3)–C(2) |
1,60 |
C(3)–C(2)–O(1) |
108 |
C(2) |
+0,153 |
|
C(4)–C(3) |
1,57 |
C(4)–C(3)–C(2) |
108 |
C(3) |
+0,463 |
|
H(5)–O(1) |
0,95 |
H(6)–C(2)–O(1) |
112 |
C(4) |
+0,145 |
|
H(6)–C(2) |
1,12 |
H(7)–C(2)–O(1) |
112 |
H(5) |
+0,201 |
|
H(7)–C(2) |
1,12 |
F(8)–C(3)–C(2) |
112 |
H(6) |
+0,018 |
|
F(8)–C(3) |
1,35 |
F(9)–C(3)–C(2) |
112 |
H(7) |
+0,018 |
|
F(9)–C(3) |
1,35 |
F(10)–C(4)–C(3) |
122 |
F(8) |
–0,209 |
|
F(10)–C(4) |
1,31 |
F(11)–C(4)–C(3) |
122 |
F(9) |
–0,208 |
|
F(11)–C(4) |
1,31 |
F(10) |
–0,137 |
||
|
F(11) |
–0,136 |
1 The numbering of atoms corresponds to Fig. 1.
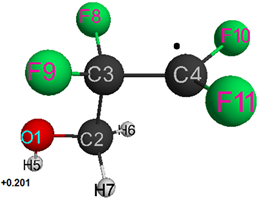
Figure 1. Geometric and electronic structure of free radical ●CF2CF2CH2OH.
Table 2. Optimized bond lengths, bond angles and atomic charges for HCF2CF2CH2O●1.
|
Bond lengths |
R, Å |
Bond angles |
Angles |
Atoms |
Atomic charges |
|
C(2)–O(1) |
1,35 |
C(3)–C(2)–O(1) |
113 |
O(1) |
–0,119 |
|
C(3)–C(2) |
1,61 |
C(4)–C(3)–C(2) |
114 |
C(2) |
+0,081 |
|
C(4)–C(3) |
1,65 |
H(5)–C(4)–C(3) |
108 |
C(3) |
+0,301 |
|
H(5)–C(4) |
1,13 |
H(6)–C(2)–O(1) |
110 |
C(4) |
+0,385 |
|
H(6)–C(2) |
1,12 |
H(7)–C(2)–O(1) |
110 |
H(5) |
+0,073 |
|
H(7)–C(2) |
1,12 |
F(8)–C(3)–C(2) |
111 |
H(6) |
+0,081 |
|
F(8)–C(3) |
1,35 |
F(9)–C(3)–C(2) |
111 |
H(7) |
+0,081 |
|
F(9)–C(3) |
1,35 |
F(10)–C(4)–C(3) |
111 |
F(8) |
–0,217 |
|
F(10)–C(4) |
1,35 |
F(11)–C(4)–C(3) |
111 |
F(9) |
–0,217 |
|
F(11)–C(4) |
1,35 |
F(10) |
–0,224 |
||
|
F(11) |
–0,224 |
1 The numbering of atoms corresponds to Fig. 2.
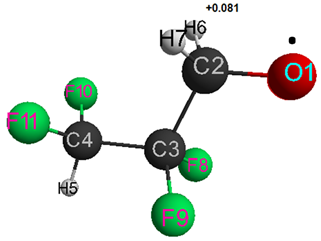
Figure 2. Geometric and electronic structure of free radical HCF2CF2CH2O●.
Table 3. Values of total energy (E0) and dipole moment (μ) of hydroxyl group of ●(CF2CF2)nCH2OH radicals.
|
Radical particle |
Е0, kJ/mol |
μ, D |
|
●CF2CF2CH2OH |
–257229 |
3,55623 |
|
●(CF2CF2)2CH2OH |
–466869 |
3,73490 |
|
●(CF2CF2)3CH2OH |
–676507 |
3,81901 |
|
●(CF2CF2)4CH2OH |
–886145 |
3,87166 |
|
●(CF2CF2)5CH2OH |
–1095783 |
3,88725 |
|
●(CF2CF2)6CH2OH |
–1305420 |
3,89884 |
Table 4. Values of total energy (E0) and dipole moment (μ) of HCF2-group for H(CF2CF2)nCH2O●radicals.
|
Radical particle |
Е0, |
μ, D |
|
HCF2CF2CH2O● |
–257095 |
1,41142 |
|
H(CF2CF2)2CH2O● |
–466741 |
1,37300 |
|
H(CF2CF2)3CH2O● |
–676378 |
1,35834 |
|
H(CF2CF2)4CH2O● |
–886016 |
1,35725 |
|
H(CF2CF2)5CH2O● |
–1095654 |
1,33629 |
|
H(CF2CF2)6CH2O● |
–1305292 |
1,33555 |
Further transformations of ●(CF2CF2)nCH2OH radicals are associated with the formation of fluoroalkoxyl radicals as:

stabilized by intramolecular interactions CF2H···●O–CH2 and CH2···F2C. These radical particles have a linear structure and differ from their non-fluorinated counterparts in that the tetrahedral configuration is preserved. The terminal radicals are characterized by a line with splitting between the components of 440 E in theoretical EPR spectra (at 77 К), which were modeled using the SIMFONIA program (Bruker, Germany) [19]. For middle radicals –CF2–C●F–CF2– (that formed in a significant amount as the γ-irradiation time by 60Со rise) the line with splitting between the 210 E components is characteristic (see Table 5).
Table 5. Characteristics of paramagnetic centers formed during radiolysis (0.6 Mrad) of polyfluorinated alcohol H(CF2CF2)4CH2OH (calculation, vacuum).
|
Duration of radiolysis, s |
Free radicals |
Content, % vol. |
|
5 |
●(CF2CF2)4CH2ОН |
91 |
|
Н(CF2CF2)4CH2О● |
5 |
|
|
Н(CF2CF2)4C●HОН, –CF2–C●F–CF2–, biradicals |
4 |
|
|
201 |
Н(CF2CF2)4CH2О● |
48 |
|
–CF2–C●F–CF2– (singlet at 77 К, doublet of quintets at 300 К) |
40 |
|
|
●(CF2CF2)4CH2ОН, Н(CF2CF2)4CH2●, Н(CF2CF2)4●, Н(CF2CF2)4C●HОН, biradicals |
12 |
1 Concentration of paramagnetic centers is ~ 25· 10-18 g-1.
According to DFT method (ORCA software package [21]) the calculated value of total g-factor for HCF2CF2CH2O● radical is 2.017. It was shown that according to aggregated data for this method and the results of modeling in SIMFONIA program for conformations of polyfluoroalkoxyl Н(CF2CF2)4CH2О● radical (note that more than 360 conformations were involved in this calculation, taking into account various torsion angles and rotation of particle structural fragments) for its linear configuration the total energy is Е0 = –54837.076 eV and g = 2.031 (total g-factor for Н(CF2CF2)4CH2О● is g = 2.016, and the value Е0 = –54837.436 eV).
Conclusion
Using a combination of classical quantum chemistry methods (MNDO, DFT) and modeling of EPR spectra (by SIMFONIA program), the structure of paramagnetic centers generated from polyfluorinated alcohols has been determined. It is shown that at initial stage the free-radical ●(CF2CF2)nCH2ОН and Н(CF2CF2)nCH2О● particles with linear structure (line with 440 E splitting) are formed, stabilization of which is promoted by intramolecular proton-donor and proton-acceptor interactions.
References
- Scheirs, J. Modern Fluoropolymers: High Performance Polymers for Diverse Applications (Wiley Series in Polymer Science) / J. Scheirs. Wiley, 1997, 660 p.
- Ameduri B., Boutevin B. Well Architectured Fluoropolymers: Synthesis, Properties and Applications. - Amsterdam: Elsevier, 2004, 508 р.
- Ameduri, B. Fluorinated Polymers: Volume 1: Synthesis, Properties, Processing and Simulation (Rsc Polymer Chemistry) / B. Ameduri, H. Sawada, T. Narita. - Royal Society of Chemistry, RSC Publishing, 2016, 439 p.
- Ameduri, B. Fluorinated Polymers: Volume 2: Applications (Polymer Chemistry Series) (Rsc Polymer Chemistry) / B. Ameduri, H. Sawada, T. Narita. - Royal Society of Chemistry, RSC Publishing, 2016, 396 p.
- Smith, D. W. Handbook of Fluoropolymer Science and Technology / D. W. Smith, S. T. Iacono, S. S. Iyer. - John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, 646 p.
- Hougham, G. G. Fluoropolymers 1: Synthesis (V. 1) / G. G. Hougham, P. E. Cassidy, K. Johns, T. Davidson. - Springer Science & Business Media, 2006, 330 р.
- Hougham, G. G. Fluoropolymers 2: Properties (Topics in Applied Chemistry) (V. 2) / G. G. Hougham, P. E. Cassidy, K. Johns, T. Davidson. Springer Science & Business Media, 1999, 408 р.
- Ebnesajjad, S. Fluoropolymer Applications in the Chemical Processing Industries: The Definitive User's Guide and Handbook (Plastics Design Library) / S. Ebnesajjad, P. R. Khaladkar. - Elsevier. William Andrew. 2017, 452 p.
- Kudashev, S. V. Methods of introducing poly- and perfluorinated fragments in to a macromolecular system (Review), Fluorine notes, 2020, 3(130), 3-4, http://www.notes.fluorine1.ru/public/2020/3_2020/article_2.html.
- Modification of Polycaproamide by 1,1,5-Trihydroperfluoropentanol / I. A. Novakov, N. A. Storozhakova, A. P. Krasnov, V. B. Ivanov, V. V. Priymak, Polymer Science. Ser.B, 2005, 47(11-12), 335-338.
- Features of Structural Transformations and Properties of Polycaproamide Modified with Polyfluorated Alcohol Immobilized on Montmorillonite / S. V. Kudashev, I. А. Zvereva, М. V. Chislov, V. M. Shapovalov, A. M. Valenkov, N. V. Kuznetsova, Russian Journal of Applied Chemistry, 2020, 93(6), 854-860.
- Reducing the Combustibility of Polycaproamide Using a Mixture of Polyelemental Flame Retardants / S. V. Kudashev, V. M. Shapovalov, A. M. Valenkov, V. N. Arisova, A. I. Bogdanov, V. F. Zheltobryukhov, Fibre Chemistry, 2020, 51(5), 346–349.
- Kudashev, S. V. Study of Ozone Aging of Fluorine-Containing Polydienurethane Elastomers / S. V. Kudashev, V. P. Medvedev, O. O. Tuzhikov , Protection of Metals and Physical Chemistry of Surfaces, 2019, 55(2), 359-362.
- Investigation of Supermolecular Structure of Fluorine-Containing Polyethyleneterephthalate Monofibers / S. V. Kudashev, T. E. Sukhanova, P. N. Yakushev, V. V. Rоdaev, V. M.Vasyukov, V. N. Arisova, A. I. Bogdanov, Fibre Chemistry, 2018, 50(1), 19-23.
- Muromtsev, V. I. Electron Spin Resonance Spectra of Fluorocarbon Radicals / V. I. Muromtsev, R. A. Asaturyan, I. G. Akhvlediani, Russian Chemical Reviews, 1971, 40(2), 175-183.
- Degradation of gamma-irradiated linear perfluoroalkanes at high / S. R. Allayarov, S. V. Konovalikhin, Y. A. Olkhov, V. E. Jackson, L. D. Kispert, D. A. Dixon, D. Ila, U. Lappan, Journal of Fluorine Chemistry, 2007, 128(6), 575-586.
- Granovsky, A. A., Firefly version 8, 2013. http://classic.chem.msu.su/gran/firefly/index.html.
- M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. J. Su, T .L. Windus, M. Dupuis, J. A. Montgomery. General Atomic and Molecular Electronic Structure System, Journal of Computational Chemistry, 1993, 14, Р. 1347-1363. Doi:10.1002/jcc.540141112.
- Programs for Modeling EPR Spectra. Access Mode: https://www.bruker.com/ru/products/mr/epr/epr-software/simulation-suites.html.
- B. M. Bode, M. S. Gordon. MacMolPlt: A graphical user interface for GAMESS // Journal of Molecular Graphics and Modelling, 1998, 16(3), 133-138.
- New ORCA Release: ORCA 4.2.1, https://orcaforum.kofo.mpg.de/app.php/portal.
ARTICLE INFO
Received 03 November 2020
Accepted 09 November
Available online December 2020
Recommended for publication by Prof. S.M. Igumnov
Fluorine Notes, 2020, 133, 1-2
