Received: October 2020
DOI 10.17677/fn20714807.2020.05.02
Fluorine Notes, 2020, 132, 3-4
A NEW PERSPECTIVE ON THE MCLAFFERTY REARRANGEMENT IN THE SPECTRA OF n-CARBOXYLIC ACIDS AND THEIR METHYL AND 2,2,2-TRIFLUOROETHYL ESTERS
N.D. Kagramanov
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991, GSP-1, Moscow, B-334, Vavilov St. 28
e-mail: ndkagram@gmail.com
Abstract: The reason that ignited the interest in this topic, was the verification of the proposed algorithm for the fragmentation of n-alkanes, under the conditions of the rearrangement processes occurring in the spectra of n-carboxylic acids of their methyl esters, and 2,2,2-trifluoroethyl esters presented in the NIST libraries. The analysis of the homologues spectra of acids C5-C34, methyl C5‑C61 and trifluoroethyl esters C6‑C22 confirmed, that the fragmentation of their alkyl chain occurs without rearrangements up to the ion [C4H7O2]+ with m/z 87 in the spectra of acids, ions [C5H9O2]+ with m/z 101 and [C6H8F3O2]+ with m/z 169 in the spectra of methyl and trifluoroethyl esters.
In contrast to the previous detachments, the detachment of ethylene from the ions with m/z 87, m/z 101, and m/z 169 is accompanied by the appearance of two ions: a rearrangement ion, and a more stable fragmentary rearrangement ion, with the loss of a hydrogen atom. Rearrangement ions with m/z 61, 75, and 143 are produced from ions 87, 101, and 169, as a result of coordination interactions between the oxygen of the carbonyl group and two hydrogen atoms of the terminal ethylene group, and the decay of the transition state, with the release of acetylene and the addition of two hydrogen atoms to the new ion. Ion pairs are formed, in the spectra of carboxylic acids C5-C30 of their methyl and trifluoroethyl esters: CH2=C(HO+H)(OH) with m/z 61, and .CH2C =(O+H)(OH) c m/z 60; CH2=C(HO+H)(OCH3) with m/z 75, and .CH2C =(O+H)(OCH3) c m/z 74; CH2=C(HO+H)(OCH2CF3) with m/z 143, and .CH2C=(O+H)(OCH2CF3) with m/z 142. The ratio of the peak intensities of rearrangement and fragmentary rearrangement ions with m/z 61 and 60; 75 and 74; 143 and 142 increases with an increase in the molecular weight of the homologue, as a result of a decrease in the vibrational excitation of M+..
Keywords: McLafferty rearrangement, mass spectrometry of carboxylic acids, ion series.
Introduction
For the first time, the intramolecular rearrangements occurring during the photolysis of dialkyl ketones: n-AmCOMe, sec-BuCOMe and n-PrCOMe, with the formation of mixtures of acetone and 1-butene, EtCOMe and ethylene, acetone and ethylene, respectively, were reported in the work of Nicholson A.J.S. [1]. The rearrangement, known as the McLafferty rearrangement, was discovered by Fred Warren McLafferty in the mass spectra of aliphatic ketones, carboxylic acids, acid esters and a wide variety of compounds, containing unsaturated groups C=O, C=N, C=C, C=S with strong electron-withdrawing properties [2]. In the spectra of carbonyl compounds, the rearrangement is regioselective, and is dependent on the presence of at least three consecutively connected CH2 groups in the chain, the presence of the carbonyl group of the H atom in the γ-position relative to the oxygen, and the distance between the atoms should not exceed 0.18 nm [3]. In the mass spectrum of n-butanoic acid C3H7-COOH, the rearrangement of the molecular ion occurs with the detachment of ethylene, through a hexatomic transition state (Fig. 1) [2].
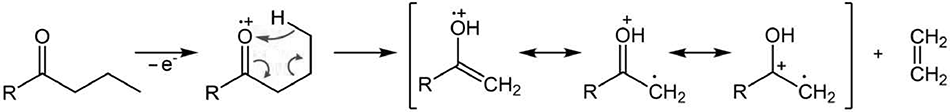
Figure 1. McLafferty rearrangement pattern (R = OH, OCH3).
The rearrangement is confirmed by the characteristic base ion .CH2C=(O+H)(HO) with m/z 60 different from the "expected" ion .+M - .C2H5 -> +CH2C=O(HO) with m/z 59.
In the spectra of C5, valeric and isovaleric acids, a noticeable peak of a doubled rearrangement - two hydrogen atoms with m/z 61 [4,2] is formed, along with an intense peak with m/z 60. A double rearrangement is differs from a rearrangement of a single hydrogen atom, in that a radical is formed instead of a molecule [2]. If a molecular ion participates in the rearrangement of butanoic acid, with the migration of a single H atom and the detachment of ethylene, then in the case of higher carboxylic acids, uncertainty arises as to which of the carbon atoms is detached from one or two hydrogen atoms and which olefin is formed in this case.
Is hydrogen abstracted, just as in butanoic acid, through a six-membered transition state, or is it by a mechanism involving the migration of H-atoms from more distant positions by a shift to carbonyl? A number of assumptions were made about the appearance of rearrangement ions, the masses of which correspond to the usual expected ions, but the peaks have increased intensity, indicating their high stability and, therefore, the possibility of intramolecular rearrangement without changing the mass. «Starting with normal acids of composition C10, homologous ions with m/z 129 CH2=CH-(CH2)3-C=O+H(OH) appear in the spectra, which, like the ions RCH=CR1-C=O+H(OH) may be a result of the initial shift of the same hydrogen atom»[5]. The formation of ions with an increased intensity was explained by nonspecific migrations of hydrogen (type P), through transition states, when the size of the transition state cycle is not determined and is not significant [6]. «In the mass spectra of methyl esters of long-chain carboxylic acids R(CH2)nCOOCH3 (n> 5), there are intense peaks of homologous ions +(CH2)nCOOCH3. The most intense peak (> 50%) is given by an ion with m/z 87 (n=2). The structure +CH2CH2COOCH3 cannot explain such a high intensity in any way. As it was shown, the ion m/z 87 is obtained, through the mechanism, presented below, by the initial long-distance migration of the H-atom to the carbonyl group, followed by the reverse migration of the mobile hydrogen atom from α- positions with respect to the carbomethoxyl group to the formed radical center and, finally, by breaking the bond according to the B1 (or A2) type: (a diagram is presented). The ion with m/z 87 +CH2CH=C(OH)OCH3 is certainly more stable than the +CH2CH2COOCH3 ion» [6]. A similar reasoning for the high intensity of the peaks of homologous ions, in the spectra of methyl esters of carboxylic acids (every four methylene units), the formation scheme (8.67а), and the structure of the ion with m/z 87 CH2=CH‑C=O+H(OCH3) are presented in the monograph [7]. Regarding the fragmentation of n-carboxylic acids in the same review, on page 261, the author notes that, «the longer the alkyl chain, the higher the intensity of the peak of the ion with m/z 61, formed during the migration to the carboxyl group of two hydrogen atoms of the alkyl chain» [7].
The probable schemes for the formation of discussed rearrangement ions, as well as the reasons for the increased intensity of homologous ions, are considered based on a detailed analysis of the spectra of carboxylic acids, their methyl esters, and 2,2,2-trifluoroethyl esters, performed according to the n-alkane fragmentation algorithm [8].
Fragmentation of C4-C34 homologues of n-carboxylic acids
By analyzing the spectra of n-alkanes, it was found that their spectrum is the sum of two main series of ions formed in parallel, that form with the removal of methyl and ethyl radicals and subsequent emissions of ethylene molecules [8].
Table 1 shows: the informative part of the spectrum of n-pentadecanoic acid (NIST Library), as well as the mass numbers of it's spectrum, emulated by the n-alkane algorithm for .CH3 and .C2H5 detachments. The spectrum was emulated, in order to confirm that the real spectrum of n-pentadecanoic acid is indeed the sum of two independent series of ions, which make it possible to distinguish between a series where rearrangement occurs with the formation of ions with m/z 61 and 60, and a series where those ions are not formed.
Table 1. Mass spectrum of n-pentadecanoic acid C15H30O2 and mass numbers of it's emulated spectrum.
Mass Spectrum CH3[CH2]14COOH [NIST Library]
|
45 11,6% |
60 93,1% |
61 22,8% |
73 100% |
87 21,8% |
101 9,7% |
115 14,5% |
129 44,1% |
143 16,4% |
157 9,2% |
171 8,1% |
185 12,2% |
199 18,6% |
213 3,7% |
227 0,1% |
242 15,9% М |
Mass numbers of the spectrum of n-C15H30O22, emulated during the primary detachment . CH3.
|
-1 |
-26 |
-28 |
-28 |
-28 |
-28 |
-28 |
-15 |
Weight |
|
- H |
- HCCH |
- H2C=CH2 |
- H2C=CH2 |
- H2C=CH2 |
- H2C=CH2 |
- H2C=CH2 |
. CH3 |
242 |
|
60 |
61 |
87 |
115 |
143 |
171 |
199 |
227 |
m/z |
Mass numbers of the spectrum of n-C15H30O2, emulated at the primary detachment . C2H5.
|
-28 |
-28 |
-28 |
-28 |
-28 |
-28 |
-29 |
Weight |
|
- H2C=CH2 |
- H2C=CH2 |
- H2C=CH2 |
- H2C=CH2 |
- H2C=H2C |
- CH2=CH2 |
. H2C-CH3 |
242 |
|
45 |
73 |
101 |
129 |
157 |
185 |
213 |
m/z |
Mass numbers of the spectrum of n-pentadecanoic acid,
emulated during the detachmnets of . CH3 and . C2H5.
|
45 |
60 |
61 |
73 |
87 |
101 |
115 |
129 |
143 |
157 |
171 |
185 |
199 |
213 |
227 |
242 |
The comparison of the mass spectrum of n-pentadecanoic acid with the mass numbers of it's spectrum, emulated according to the algorithm of parallel primary detachments of . CH3 and .C2H5, with subsequent emissions of ethylene (Table 1), confirms their complete similarity, and the formation of rearrangement ions with m/z 61 and 60 only in only one of the series.
The graphs of the intensities of the main peaks of the mass spectra of homologues of C2-C30 n-carboxylic acids, presented in (Fig. 2) illustrate their dependence on the molecular weight of
60‑452 Da.
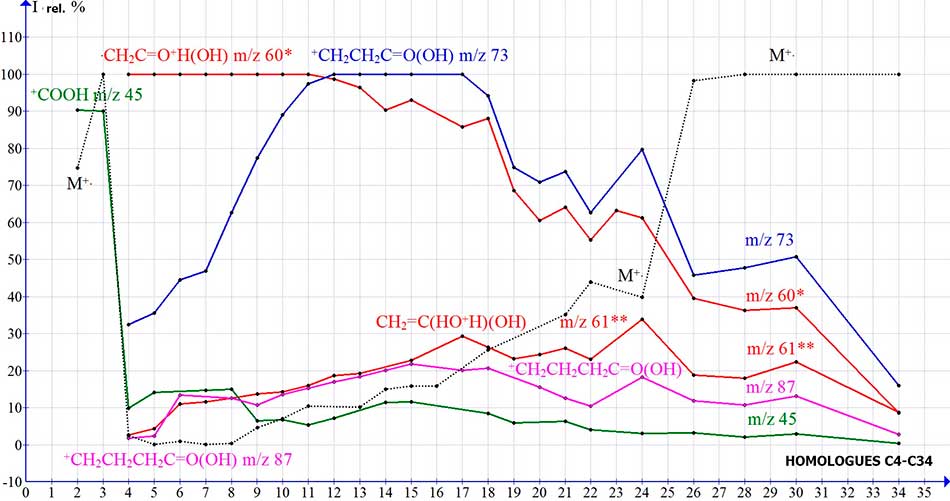
Figure 2. Dependences of the intensities (I rel.%) of the peaks of n-carboxylic acid ions on their molecular weight.
High intensities M+. C2-C3 75% and 100% are not associated with rearrangement, as well as the basic molecular peak of C1 n-alkanes, M+. C1-C2 n-alkyl chlorides and n-alkyl bromides [8,9] are the result of the strength of the structures of the first homologues and the minimal possibility of fragmentation, in comparison with the structures of the following homologues and the possibilities of their fragmentation. The reason for the high intensity of M+. peaks of acids C2-C3 is the optimal ratio of the masses of their acyl chain and hydroxycarbonyl groups 1: 3, and 1: 1.6. Starting with the C9, the M+. intensity increases, as a result of a decrease in vibrational excitation, with an increase in molecular weight. The decrease in the intensity of the peaks of fragment ions of homologues C17-C34, is probably the result of an increase of the energy deficit, required for complete fragmentation of M+..
Rearrangement of C4 butanoic acid occurs through the 6-membered transition state of the molecular cation radical (Fig. 1), with the migration of one hydrogen atom of the C2H5 group to the oxygen carbonyl, and the release of an ethylene molecule [2]. Along with the basic rearrangement ion with m/z 60, the spectrum contains an ion with m/z 61 with the peak intensity of 2.6%, which corresponds to the intensity of the isotopic C13 peak of 2.36%. Another decay process of M+. C4 is a breakaway .CH3, occurring in a non-rearrangement series, with the formation of the ion +CH2CH2C=O(OH) m/z 73 32.4%, fragmenting with the release of С2H4 and the formation of the +COOH ion. With an increase in molecular mass in the spectra of C11-C17 homologues, peak 73 becomes basic, and in the spectra of C18-C34 its intensity begins to decrease, the same way as the intensity of the rearrangement peak with m/z 60 (Fig. 2). The high peak intensity of the ion with m/e 73 made it possible to assume that, either it's structure does not correspond to +CH2CH2C=O(OH), and due to the shift of the hydrogen atom, the ion rearranges into CH2=CH-C=O+H(OH), or that there is another, alternative reason of it's high intensity.
The C5 homologue has a peak intensity with m/z 61 of 4.4%, which is twice the intensity of the C13 isotopic peak, while the C11 homologue is 16%. In the spectra of C5-C34, the rearrangement ion with m/e 61, is formed from a fragment ion with m/z 87, through a transition state, with the migration of two hydrogen atoms of the terminal ethylene group to the carbonyl oxygen with the release of an acetylene molecule (Fig.3).
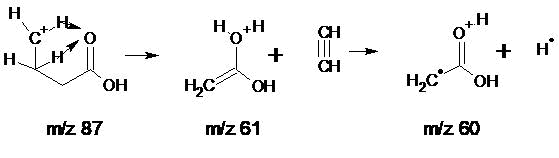
Figure 3. Scheme of formation of rearrangement ions: С2H5O2 with m/z 61 and its fragmentary rearrangement ion С2H4O2 with m/z 60.
The energies of ethylene, acetylene, vinyl radical and H atoms detachment from the terminal ethylene group of the ion with m/z 87, can be approximately estimated, by comparing the values of the standard enthalpies of formation of the detached fragments (Table 2).
In the C5 spectrum, the direct transformation of an ion with m/z 87 into a rearrangement ion with m/z 60 is possible, when one hydrogen atom of the terminal ethylene group migrates to the carbonyl oxygen, with the detachment of the vinyl radical ΔHf 0 = 70.5 kcal/mol.
In the mass spectra of n-alkanes, one of the most energy-consuming processes is the detachment of hydrogen atoms, the formation enthalpy of which is ΔHf 0 = 52.1 kcal/mol.
Table 2. Enthalpies of formation ΔHfo [10*].
|
CH2=HC. |
C2H2 |
.H |
C2H4 |
C3H6 |
C4H8 |
|
|
ΔHf0 (g), 298K (kcal/mol) |
70,5* |
54,2 (ref.) |
52,1* |
12,5 (ref.) |
4,88 (ref.) |
-0,03 (ref.) |
To detach two hydrogen atoms from ethylene with the formation of acetylene, it is necessary to spend 54.2 kcal/mol, which almost coincides with the enthalpy of a hydrogen atom formation of 52.1 kcal/mol. In comparison with the enthalpy of ethylene, the minimum value of the enthalpy of С4H8 formation deserves attention.
The ratio of the intensities of the peaks of ions with m/z 61 and 60, varying with increasing molecular weight of the homologues, allows us to conclude that the strength of the two hydrogen atoms bonds, coordinated by the carbonyl group differ. The ion with m/z 61 CH2=C-O+H2(OH), formed as a result of the acetylene detachment from the +(CH2)3COOH ion, is less stable than it's fragment ion with m/z 60 .CH2C=O+H(OH). However, with an increase in molecular weight and a decrease in excess excitation, the intensity of the peak with m/z 61 increases, and in the C34 spectrum the intensities of the peaks with m/z 61 and 60 are the same.
Figure 4 shows the mass spectrum of pentadecanoic acid and a graph of the two main series of ions, with real peak intensities.
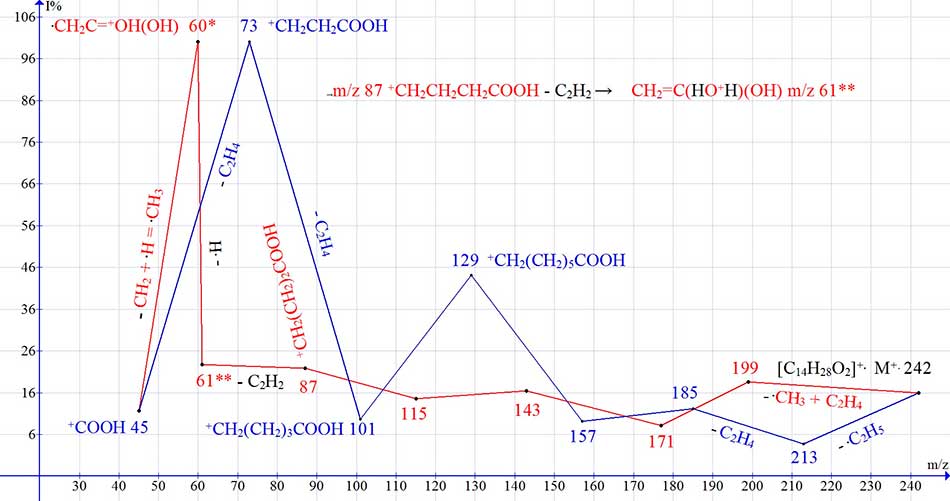
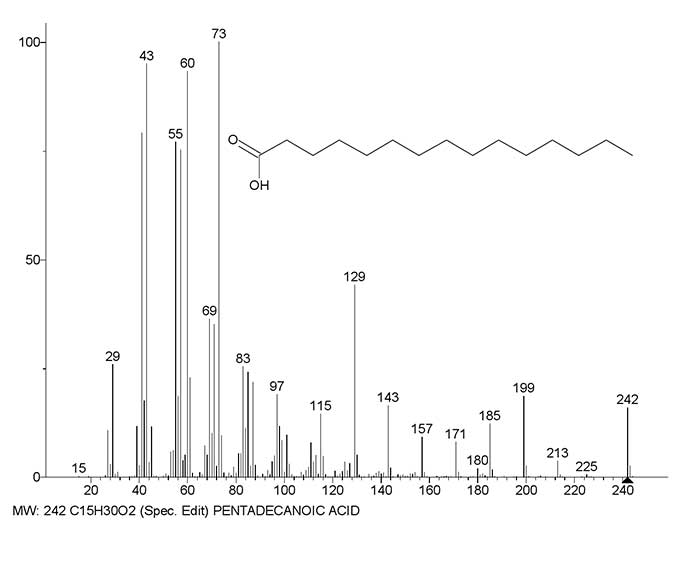
Figure 4. Mass spectrum of pentadecanoic acid C15H30O2 and a graph of two spectrum ions series, with real intensities of the peaks.
In the series that leads to rearrangement ions with m/z 61 and 60 (Fig. 4 series red), all the previous peaks: 199, 171, 143, 115, 87, that differ in ethylene groups, have similar intensities, of approximately 20%. In the series leading to the ion with m/z 73 (Fig. 4 series blue), the peaks with m/z 185, 129 and 73, that differ in butylene groups, have higher intensities of 12, 44 and 100%. With homologues increasing in the molecular mass of, the number of intensity peaks, differing by the C4H8 group increases. There are five such ions in the spectrum of C22: 297, 241, 185, 129, and 73. Based on the increased intensity of the peaks, there is no reason to assume that these five ions are rearrangement ions.
Compared to the detachments of C2H4, the detachment of C4H8 leads to a doubled removal of excess energy, and an increase in the number of such detachments contributes to a greater stabilization of the formed ions and an increase in the intensity of their peaks. Thus, along with the "slow" (C2H4 detachments) rearrangement series (Fig. 4, series red), a "fast" (C4H8 detachments) non- rearrangement series (Fig.4 series blue) is formed.
Fragmentation of acid homologues of with a tertiary carbon atom in position 3 (Fig. 5, 6) occurs the same way as in the spectra of n-carboxylic acids, with the detachment of acetylene and the formation of two rearrangement ions with m/z 61 and 60. However, in acids with a tertiary carbon atom and two alkyl chains, the number of possible C4H8 detachments is less than in n-carboxylic acids with the same number of carbon atoms, which is probably the reason behind a decrease in the intensity of the peak with m/z 73.
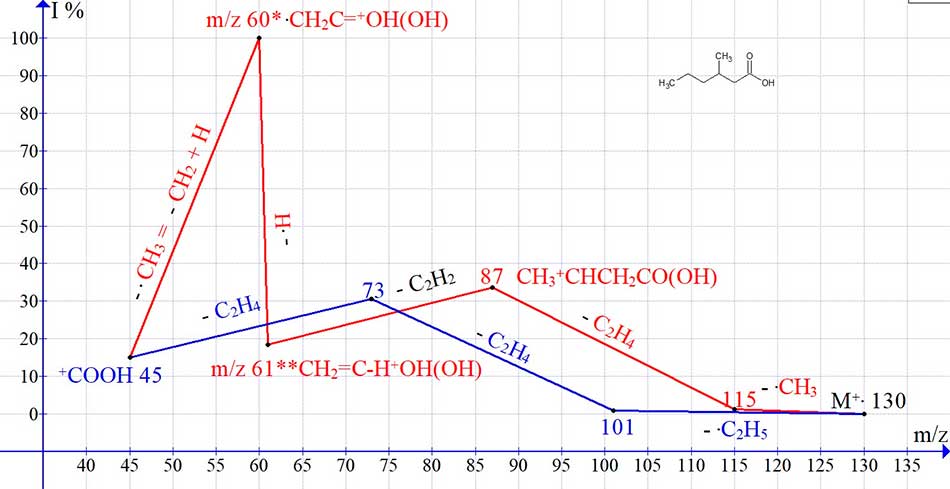
Figure 5. Graph of two ion series in the spectrum of C7H14O2, with real peak intensities.
Fragmentation of 3-ethylheptanoic acid occurs in a more complex manner (Fig.6).
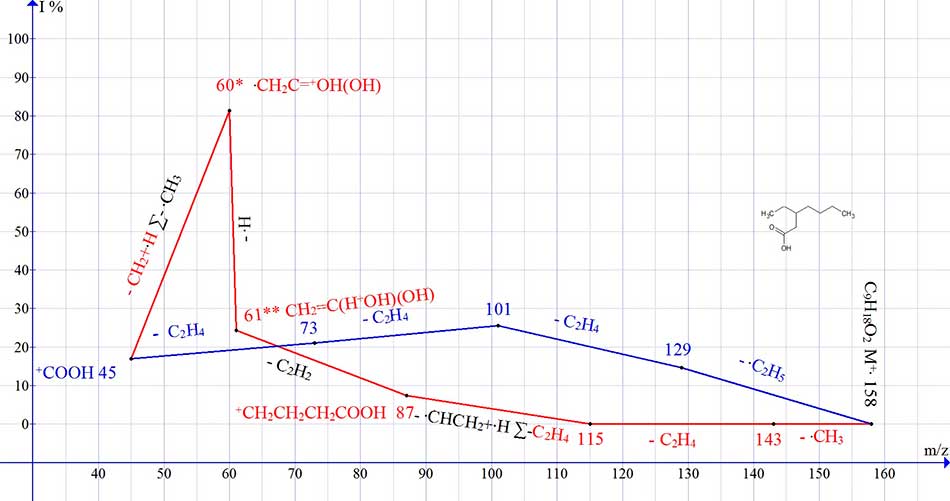
Figure 6. Graph of two ion series of the C9H18O2 spectrum, with real peak intensities.
In the С9H18O2 spectrum, after the detachment (from the С4 chain) of the methyl radical, and the ethylene molecule (Fig. 6, series red), the vinyl radical .СH-CH2, including the tertiary carbon atom, is released. This leads to the rearrangement of the chain and an additional detachment of the hydrogen atom of the terminal methyl group (which in total is equal to the detachment of ethylene) with the formation of the +CH2CH2CH2COOH ion, with m/z 87. The ion with m/z 87 fragments further to form a rearrangement ion with m/z 61, and it's fragment ion with m/z 60.
Rearrangements of acid homologues with a tertiary carbon atom in position 2 (for example, С20H40O2) are hindered by the absence of a stabilizing methylene group in position 2. The rearrangement series of С20H40O2 fragmentations (Fig. 7 series red) begins with the detachment of the . С2H5 radical from the small С8 chain. As a result of two subsequent detachments of C2H4, an ion with m/z 227 is formed, which fragments with the detachment of the vinyl radical, and the migration of only a single hydrogen atom to the carbonyl group. The subsequent decay of the rearrangement ion with m/z 200* with the repeated emission of the vinyl radical from positions 2-3 and the hydrogen atom, that is, the ethylene molecule, leads to an ion with m/z 172. Then, the terminal C2H5 group is detached, followed by ethylene fragmentation with the formation of an ion with m/z 87, and the detachment of the vinyl radical with the formation of a rearrangement ion with m/z 60.
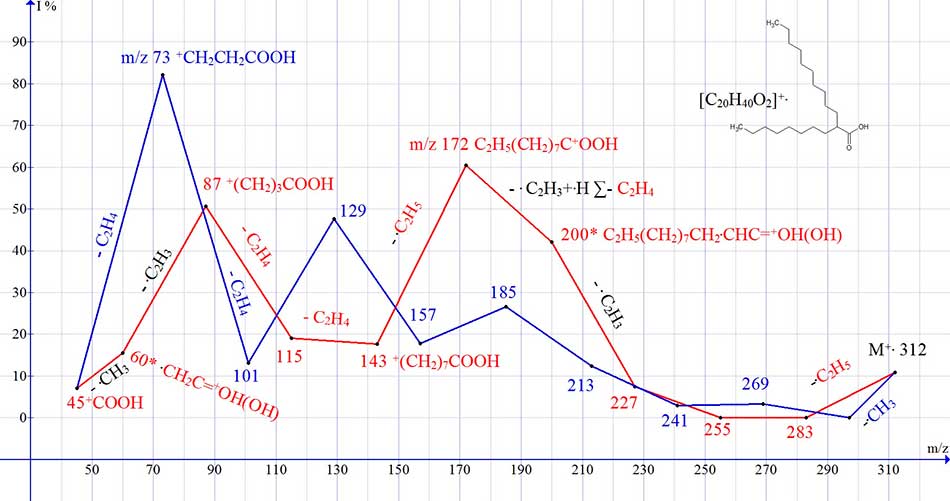
Figure 7. Graph of two ions series in the spectrum of C20H40O2, with real peak intensities.
In the non-rearrangement series (Fig. 7, series blue), the intensity of the ion peak with m/z 73 82% is the result of 3 C4H8 detachments, with the formation of peaks with m/z 185,129 and 73 increasing in intensity.
Thus, the following rearrangement ions are formed in the spectra of carboxylic acids:
- fragment rearrangement ion with m/z 60 . СH2C=(O+H)(OH) of butanoic acid C4 100%, formed by the detachment of one hydrogen atom from the ethyl group of the molecular ion CH3CH2CH2CO+(OH) with the release of ethylene;
- fragment rearrangement ion with m/z 61 CH2=C(HO+H)(OH) in the spectra of C5-C30 homologues 7.4% - 22.0%, formed when two hydrogen atoms are detached from the terminal ethylene group of the fragment ion with m/z 87 +CH2CH2CH2COOH with acetylene release;
- fragment rearrangement ion with m/z 60 .CH2C=(O+H)(OH) homologues C5-C30100%-37%, formed by the detachment of one hydrogen atom from a rearrangement ion with m/z 61.
During fragmentation of 3-ethylheptanoic acid C7, an ion with m/z 115, including a tertiary carbon atom, emits a vinyl radical . CH-CH2. This leads to the rearrangement of the chain and additional detachment of the hydrogen atom of the terminal methyl group (which in total is equivalent to the detachment of ethylene), with the formation of the +CH2CH2CH2COOH ion with m/z 87. When acetylene is detached from the ion with m/z 87, a rearrangement ion with m/z 61 18.5% CH2=C(HO+H)(OH) appears.
Rearrangements of acid homologues with a tertiary carbon atom in position 2 (for example, С20H40O2) are hindered by the absence of a stabilizing methylene group in the 2-position. As a result of two detachments of C2H4, an ion with m/z 227 is formed, which fragments, instead of the acetylene release, with the detachment of the vinyl radical and the migration of only one hydrogen atom to the carbonyl group.
Fragmentation of C4-C61 homologues of methyl esters of n-carboxylic acids
The McLafferty rearrangement in the methyl ester spectrum of the C5 homologue begins with the detachment of ethylene from the molecular ion CH3CH2CH2CO+.(OCH3) (Fig. 8).
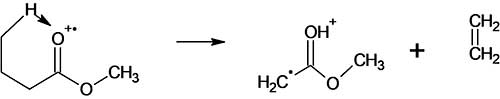
Figure 8. Scheme of formation of the rearrangement ion С3H6O2 with m/z 74.
If the intensity of the peak of the ion with m/z 75 2.7% corresponds to the intensity of the isotopic peak C13 in the spectrum of methyl ester of butanoic acid, then for homologues C26-C29 (Fig. 9) it increases to 37-48%.
An increase in the intensity of the peak with m/z 75 confirms the formation of a rearrangement ion with two coordinated hydrogen atoms СH2=C(HO+H)(OCH3) as a result of the release of acetylene by the +CH2CH2CH2COOCH3 ion with m/z 101.
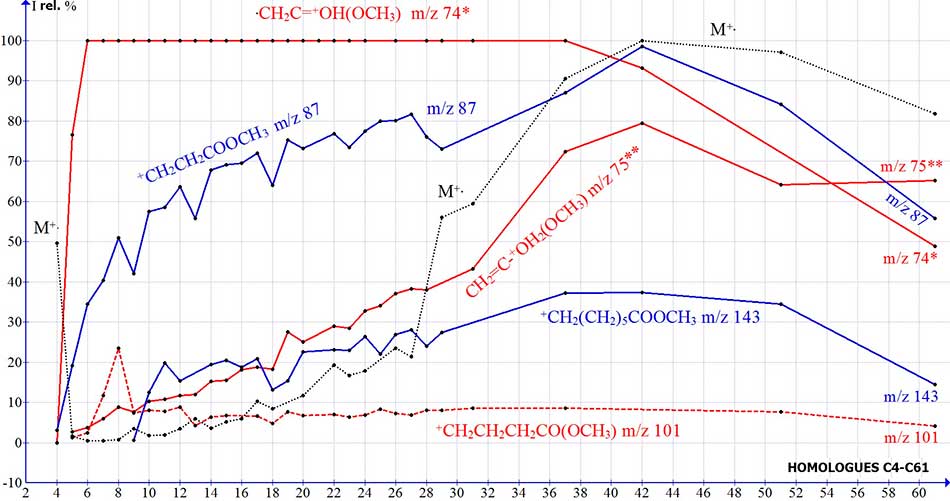
Figure 9. Dependences of the intensities (I rel.%) of the ion peaks of methyl esters of n-carboxylic acids on their molecular mass.
With an increase in the molecular mass of homologues, along with an increase in the peak intensity of the rearrangement ion with m/z 75, the intensity of the molecular ion also increases. The peak intensity of the ion with m/z 101, which is the parent ion of the rearrangement ion with m/z 75, remains constant at 8%.
Figure 10 shows the mass spectrum of octacosanoic acid methyl ester and a graph of two series of ions, with real peak intensities.
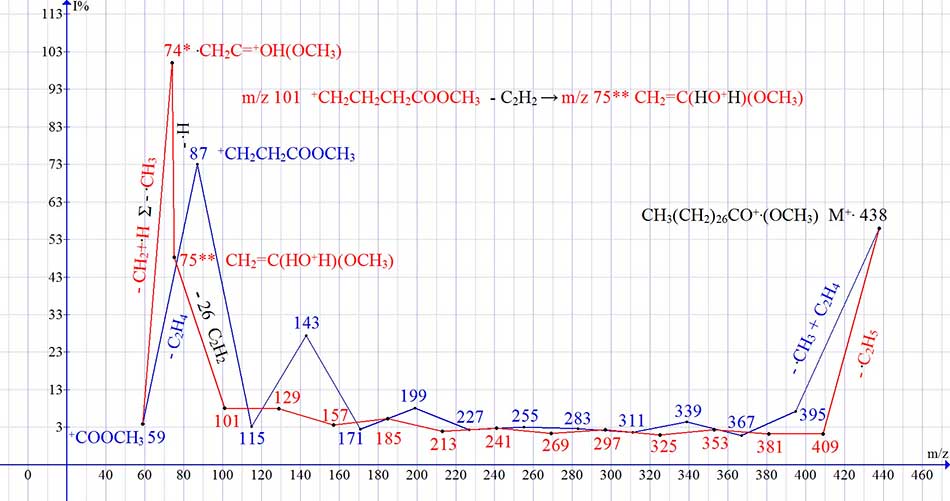
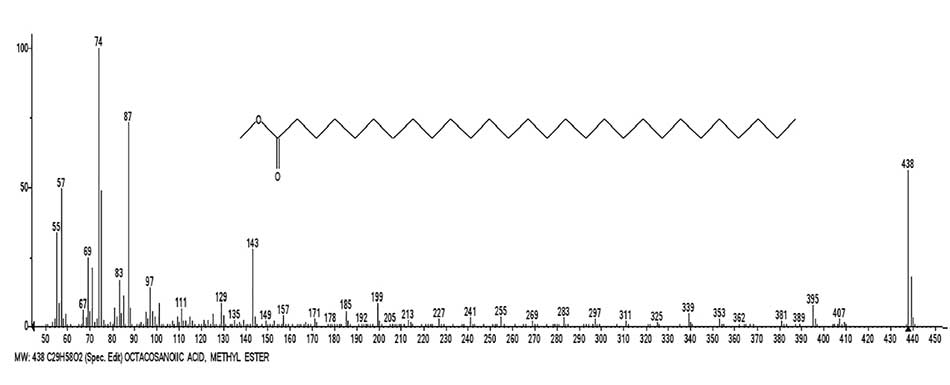
Figure 10. Mass spectrum of octacosanoic acid methyl ester С29H58O2 and graph of two series of spectrum ions, with real peak intensities.
The fragmentation of methyl esters C5-C61, just like the fragmentation of n-carboxylic acids, occurs with the detachment of acetylene and the formation of rearrangement ion with m/z 75; and it's fragment rearrangement ion with m/z 74.
As in the spectra of carboxylic acids, in the non-rearrangement series of the spectra of methyl esters (Fig. 10, series blue), intense ions with m/z 87, 199 and 143 are formed, differing by the C4H8 group.
Fragmentation of C5-C22 homologues of 2,2,2-trifluoroethyl esters of n-carboxylic acids
The dependences of the peak intensities of the ions of trifluoroethyl esters on their molecular mass (Fig. 11) differ from similar dependencies for n-carboxylic acids (Fig. 2) and their methyl esters (Fig. 9), in that they have a more gentle character.
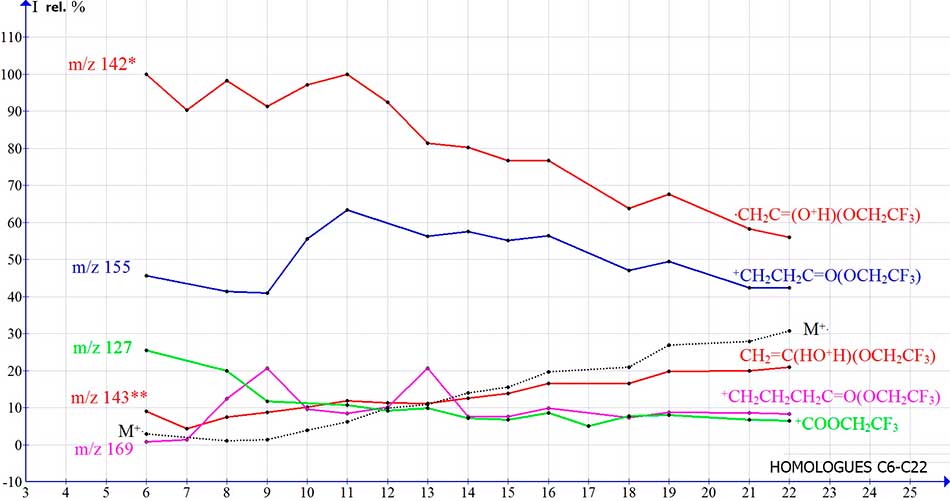
Figure 11. Dependencies of the peak intensities (I rel.%) of 2,2,2-trifluoroethyl esters of n-carboxylic acids ions, on their molecular weight.
This is due to the fact that, on the one hand, the range of the presented spectra of homologues of trifluoroethyl esters is significantly smaller, than in the case of carboxylic acids, and on the other hand, since the mass of trifluoroethyl ester is 82 Da (CHCF3 group) more than the mass of n-carboxylic acid, it is likely that an increase in the masses of homologues to CH2 groups will have a greater effect on the fragmentation of n-carboxylic acids than their trifluoroethyl esters.
Figure 12 shows the mass spectrum of eicosanoic acid 2,2,2-trifluoroethyl ester, and a graph of two series
of spectrum ions, with real peak intensities.
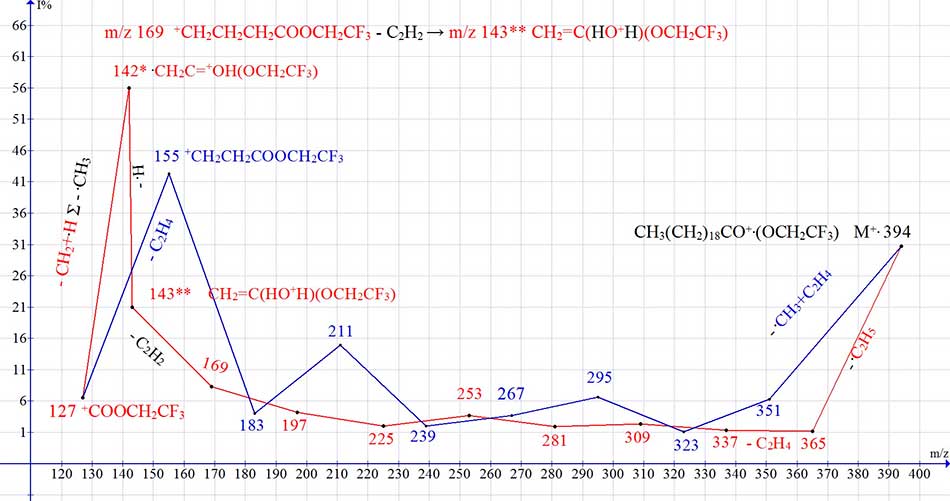
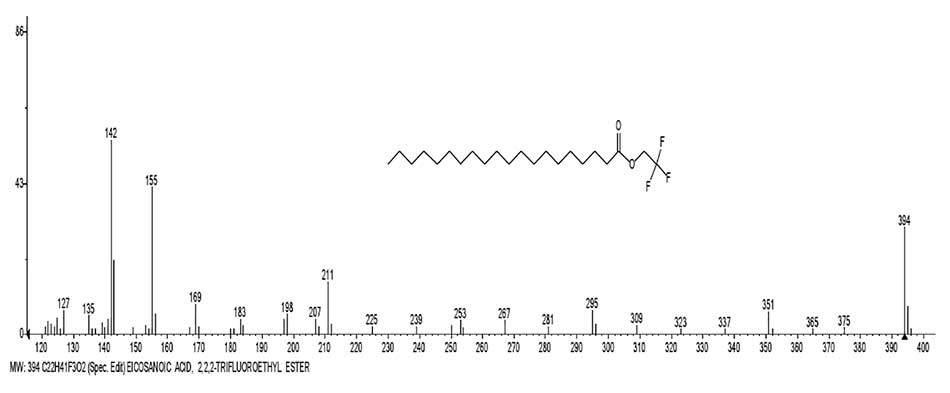
Figure 12. Mass spectrum of 2,2,2-trifluoroethyl ester of eicosanoic acid C22H41F3O2, and a graph of two series of spectrum ions, with real intensities of peaks.
In trifluoroethyl esters of carboxylic acids, the CF3 group, which has a strong electron-accepting property, is separated from the carbonyl group by a methylene group, and an oxygen atom, which excludes it's influence on rearrangement. In the spectra of trifluoroethyl esters of carboxylic acids (Fig. 12), rearrangements occur the same as in the spectra of acids, with the detachment of acetylene and the formation of a rearrangement ion with m/z 143 and it's fragment ion with m/z 142.
Unlike trifluoroethyl esters of carboxylic acids, in esters of trifluoroacetic acid, the CF3 group shifts the electron density of the carbon atom of the carbonyl group to itself, weakening the neighboring CF3C=O - OR bond. This weakening of the bond quiet likely determines the composition of the CF3HC=HO+ rearrangement ion with m/z 99. The detachment of С2H2 from the CF3C=OOCH2CH2+ ion with m/z 141 does not occur, since the peak of the CF3C=O+H(OH) ion with m/z 115 has a minimum intensity of 0.9-0.3%.
Figure 13 shows the rearrangement processes of fragmentation occurring in the mass spectra of homologues C4, C5, and C9 of trifluoroacetic acid esters.
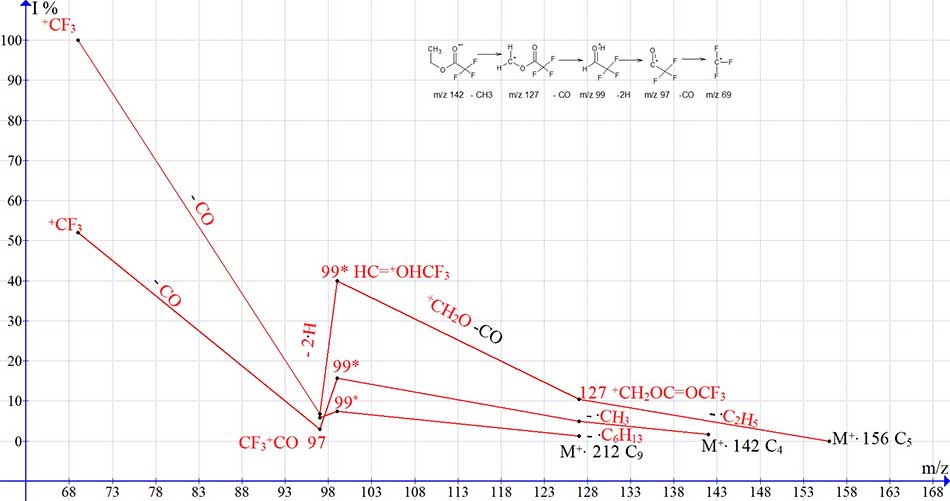
Figure 13. Rearrangement series of ions of mass spectra C4, C5 and C9 of trifluoroacetic acid esters with real peak intensities.
In the mass spectrum of the C11H19F3O2 MW 240 homologue, the mass of the C9H19 = 127 Da alkyl chain, which exceeds the mass of trifluoroacetic acid 114, can be the reason behind the change in fragmentation - the termination of rearrangement, with the formation of ions with m/z 127 CF3COOCH2+ and c m/z 99 CF3HC=O+H. As a result, the spectra of homologues C11-C22 MW 240-394 become the spectra of olefinic СnH2n, alkenyl ions СnH2n-1 and alkyl ions СnH2n+1.
Conclusions
The fragmentation algorithm proposed for n-alkanes has successfully confirmed the formation and decay sequences of rearrangement ions in the spectra of homologues of n-carboxylic acids, acids with a tertiary carbon atom, methyl and trifluoroethyl esters of n-carboxylic acids.
Ions that fragment with rearrangement, in which a six-membered transition state with oxygen of the CO group is realized:
- in n-carboxylic acids +CH2-CH2-CH2-COOH with m/z 87;
- in methyl esters of acids +CH2-CH2-CH2-COOCH3 with m/z 101;
- in trifluoroethyl esters +CH2-CH2-CH2-COOCH2CF3 with m/z 169.
In the McLafferty rearrangements, the oxygen atom of the carbonyl group coordinates the two hydrogen atoms of the terminal ethylene groups of ions 87, 101 and 169, with the release of the acetylene molecule.
As a result, two ions are formed: a rearrangement ion, and a more stable fragment-rearrangement ion with the loss of one hydrogen atom:
- CH2=C(HO+H)(OH) m/z 61 and . CH2C=(O+H)(OH) m/z 60;
- CH2=C(HO+H)(OCH3) m/z 75 and . CH2C=(O+H)(OCH3) m/z 74;
- CH2=C(HO+H)OCH2CF3 m/z 143 and . CH2C=(O+H)OCH2CF3 m/z 142.
The ratio of peak intensities of rearrangement and fragment ions with m/z 61 and 60, 75 and 74, 143 and 142 increases with an increase in the molecular weight of the homologue, as a result of a decrease in the vibrational excitation M+..
Mass spectra of n-carboxylic acids, their methyl and trifluoroethyl esters are two independent series of ions: a "fast" non-rearrangement series with prevailing C4H8 detachments, and a "slow" rearrangement series with C2H4 detachments.
High intensity of ion peaks +CH2CH2C=O(OH) with m/z 73, +CH2CH2C=O(OCH3) with m/z 87, and +CH2CH2C=O(OCH2CF3) with m/z 155, formed in non-rearrangement series - are the result of C4H8 detachments leading to a doubled withdrawal of excess energy in comparison with C2H4 detachments, and an increase in the number of such detachments leads to greater stabilization of the formed ions and an increase in their intensity.
Acknowledgments
This work was supported by Ministry of Science and Higher Education of Russian Federation using scientific equipment of Molecules Structure Study Center of INEOS RAS.
References
- Nicholson, A.J.C.,Transaction of the Faraday Society, 1954, 50, 1067.
- McLafferty, Fred W., Anal.Chem., 1959, 31, 82.
- Budzikiewicz H., Djerassi C., Williams D.H. Mass Spectrometry of Organic Compounds. San Francisco, Holden-Day Inc., 1967. 690 p.
- Happ, G.P., Stewart, D.W., J.Am. Chem. Soc. 1952, 74, 4404.
- Wolfson N.S., Zaikin V.G., Mikaya A.I., Mass spectrometry of organic compounds. M., Khimiya, 1986, 312 p., p. 228. (in Russian)
- Takhistov V.V., Practical mass spectrometry of organic compounds. Leningrad, 1977.268 p., pp. 95-96. (in Russian)
- Lebedev A.T., Mass-spectrometry in organic chemistry. Moscow, Binom, 2003. 493p., pp. 259-261. (in Russian)
- Kagramanov N.D., Fluorine notes, 2020, 1 (128), 3-4.
- Kagramanov N.D., Tyutyunov A.A. Fluorine notes, 2019, 5 (126), 5-6.
- Takhistov V.V. Organic mass spectrometry. Thermochemical description of isomerization and fragmentation of ions and radicals in the gas phase. Leningrad, Nauka, 1990, 221 p., pp. 24-25. (in Russian)
ARTICLE INFO
Received 01 October 2020
Accepted 06 October 2020
Available online October 2020
Recommended for publication by PhD M. A. Manaenkova
Fluorine Notes, 2020, 132, 3-4
