Received: August 2020
DOI 10.17677/fn20714807.2020.05.01
Fluorine Notes, 2020, 132, 1-2
NMR SPECTROSCOPY OF PRODUCTS BASED ON TETRAFLUOROETHYLENE OXIDE
Pospelova N.B.1, Mokrushin I. G.2
1 Federal State Unitary Enterprise «Russian Scientific Center «Applied Chemistry»» (Perm Branch), Russia.
2 Perm State National Research University, Perm, Russia.
Abstract: The use of fluorine NMR spectra provides a unique opportunity to control technological processes and to analyze of reactive products, a production technologies of which were developed in Perm branch of Federal State Unitary Enterprise “Russian Research Centre of Applied Chemistry”. This article describes the known methods for producing tetrafluoroethylene oxide, oligomers and products based on them. Detailed information on the control of the technological process using the method of NMR spectroscopy on hydrogen and fluorine nuclei is given.
Keywords: fluoro-19 NMR spectroscopy; polyfluorinated compounds, TFE, oxide, tetrafluoroethylene oxide.
Introduction
The receipt of various thermostable and corrosion-resistant materials that are resistant to ultraviolet and radiation exposure requires the development of methods for synthesis and analysis of compounds. Among them, the perfluorinated oxygen-containing precursors of polymer and composite materials with desired properties are in the first place. Of great importance are perfluoroalkylvinyl ethers based on oligomers of tetrafluoroethylene oxide - monomer-4 oxide (OTFE, OM-4, perfluorooxirane), which are used, for example, to obtain frost-resistant rubbers. In this article, we have analyzed a range of compounds containing basically a fragment of tetrafluoroethylene oxide (-CF2CF2O-), production of which has been mastered on the basis of Federal State Unitary Enterprise (Perm Branch) “Russian Research Centre of Applied Chemistry”. This publication gives the characteristic NMR chemical shifts (CS) (relative CFCl3) that used for analysis. The signals from "analytical" groups make it possible to confirm the presence of a compound in the mixture and to "assemble the molecule" via integral intensities of remaining parts. Earlier we have described the methods of experiments and calculations of composition [1-3]. The solvent or various compounds in the reaction mixtures contribute to the change in position of fluorine NMR line within 3-5 ppm, however, a sufficient range of shifts (~ 400 ppm) makes it possible to correctly select the analytical line and determine the ratio of identified components. The impurities in target compounds depend on the production methods. Let's consider the main known reactions of OTFE production and its oligomerization.
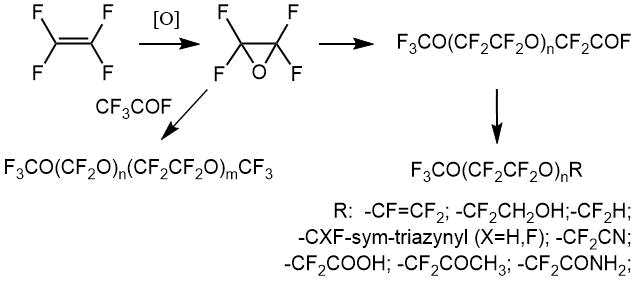
Tetrafluoroethylene oxide
Tetrafluoroethylene oxide (OTFE) is produced industrially in both gas and liquid phases. Gas-phase methods are characterized by simplicity of realization and instrumentation.
One of the gas-phase methods is the process of oxidation of tetrafluoroethylene (TFE) by molecular oxygen on silicon oxide at atmospheric pressure and at a temperature range from 0 to 50°C (with preliminary activation of gas mixture at 190-300°C [4]); in gas phase (with UV irradiation within wavelength range from 1800 to 3000 Å, within temperature range from -30 to + 150°C and at pressure of 2 atm [5-6]. As a result of this reaction (along with tetrafluoroethylene oxide), a large amount of both gaseous products (carbonyl fluoride, perfluorocyclopropane, carbonic anhydride), a liquid products (polyoxyperfluoromethylene) and a solid by-products are formed.
Previously, the process of photochemical oxidation of tetrafluoroethylene with oxygen in the presence of a source of free radicals: fluorine, chlorine, bromine, iodine, oxide and nitrogen dioxide, hydrogen bromide and trifluoromethylhypofluorite, giving gaseous free radicals in catalytic amounts, was described. Oxygen is premixed with an inert gas diluent. Free-radical reagents are taken in very small amounts, for example, the concentration of bromine in gas mixture is within the range from 4·10-5 to 6·10-5 vol.%. In order to reduce the explosiveness of this process, oxygen is premixed with an inert diluent, for example, nitrogen, in a 1:1 ratio. The ratio of oxygen to fluoroolefin is preferably equal 1: 1 [6].
Liquid-phase oxidation processes are technologically more safety.
Process of tetrafluoroethylene (TFE) oxidation by molecular oxygen in perfluorinated or chlorofluorinated solvent and in the presence of ozone within temperature range from 0 to 40°C is known. Carbonyl fluoride (COF2) and perfluorocyclopropane are formed as by-products. Also known method TFE oxidation by oxygen in the liquid phase in the presence of a source of free radicals produced in catalytic amounts. Chlorine, bromine, iodine, nitrogen oxide/dioxide and hydrogen bromide are used as a source of free radicals; and as a solvent - polyfluorinated hydrocarbons and ethers [6].
Previously, the method for liquid-phase TFE oxidation by oxygen in the presence of a source of free radicals was described, which uses fluorooxytrifluoromethane (CF3OF) or elemental fluorine, and the process is carried out within temperature range from 15 to 60 °C in freon-318 (perfluorocyclobutane). The initiator is added to the nitrogen-oxygen mixture (N2:O2 = 1:1) in an amount of 1-3 % vol. The TFE : O2 ratio is maintained at 1. Process of liquid-phase oxidation of tetrafluoroethylene by molecular oxygen in the inert fluorochlorocarbon solvent is carried out with stirring, UV irradiation and using as initiator of oxidation of bis (fluoroxy) difluoromethane [7].
In laboratory conditions, the preparation of OTFE by oxidation of TFE with molecular oxygen in the presence of molecular fluorine [8], with oxygen in gas phase within temperature range from -50 to 125 °C and UV irradiation [9] and with ozone in 1,1,2-trichlor-1,2,2-trifluoroethane at a temperature 0 °C [10-11] is described. Apparently, the reaction proceeds through the formation of mol-ozonide, followed by formation of carbonyldifluoride and difluorooxirane. Tetrafluoroethylene oxide can also be obtained by electrochemical fluorination of ethylene oxide in hydrofluoric acid [6].
It is reported that OTFE is obtained as one of radical reaction products of hexafluoro-1,3-butadiene with nitrogen oxide (IV) NO2 within temperature range from 27 to 160 °C. Among the products of olefinic carbon attack by nitrogen dioxide, CF3C(O)F, C(O)F2, NO, FNO, SiF4 (from glass) were identified. TFE can react under similar conditions [12].
Thus, various methods of tetrafluoroethylene oxide preparation have been previously studied quite well. At the site of Federal State Unitary Enterprise "Russian Research Centre of Applied Chemistry", tetrafluoroethylene oxide was obtained without intermediate isolation according to the methods [6, 7], with further conversion into target products. NMR spectroscopy was complemented by chromatographic analysis and IR spectroscopy. The composition of gas mixture from reactor was monitored by GLC method via LCM-72 apparatus, with detector-katharometer and chromatographic column 5 m long and 4 mm in diameter filled with silanized silochrome. Carrier gas (helium) is fed at a rate of 1.5-2 l/h, the column temperature is 20°C. In addition, the composition of products was identified by IR spectrum, where there is a band at 1610 cm– 1, characteristic for OTFE [6].
Oligomers of tetrafluoroethylene oxide
To give of perfluoropolyether carboxylic acid fluorides, tetrafluoroethylene gas, oxygen gas and a gaseous or liquid initiator (or initiators) are fed into reactor containing solvent in liquid phase. Alternatively, the initiator (or initiators) can be introduced in full into reactor with liquid phase before process starting. This option can be used, for example, when the initiator (or initiators) is liquid at a room temperature. Peroxidized perfluoropolyethers obtained as a result of reaction contain perfluoroxyalkylene components of formulas (СF2СF2O) and (СF2O). The molar concentrations of components (СF2СF2O) usually range from 5 to 95% (preferably from 20 to 90%). According to invention process usually produces the peroxidized perfluoropolyethers in which the ratio of end COF groups to non-functional end groups is very low; this ratio usually being less than 5% (preferably less than 2%). Number-average molecular weight of products obtained usually ranges from several hundred to several hundred thousand Da, i.e. from 500 to 100000 Da [13]. It should be noted that it is possible to industrially obtain the entire product line at one facility, both mainly acyl fluorides (hereinafter referred to as "oligomers") and "closed" polyethers [14].
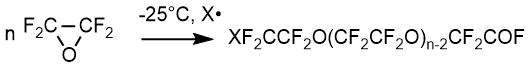
Thus, the same or different end groups represent the following radicals: XСF2-, XСF2СF2-, -CFO and -СF2СOF, where X is a fragment of initiator (or initiators) and/or solvent molecules. Typically X is F, Cl, or perfluoroalkyl or perfluoroalkoxy group. When the initiator contains two O-F bonds, this fragment can be linked to two growing polymer molecules, and it is incorporated into molecular chain of peroxidized perfluoropolyether product. Therefore, the nature of end groups varies from product to product, depending on the nature of initiator (or initiators) and solvent, as well as on the process conditions.
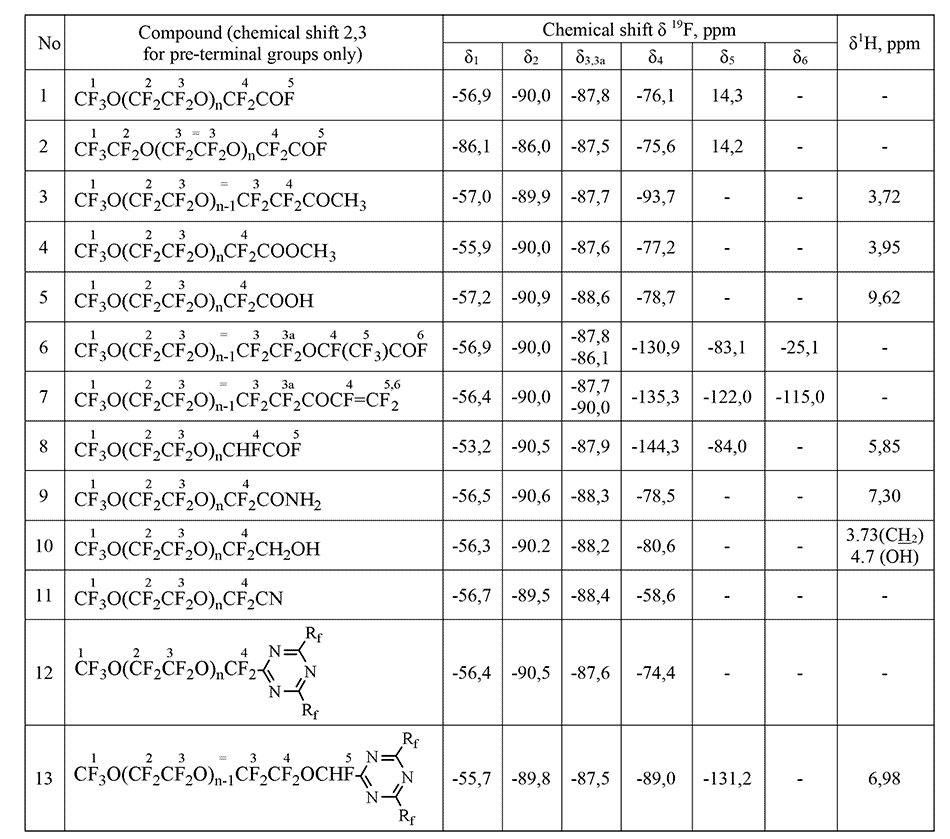
The main condition for possibility of quantitative analysis via NMR method is the presence of at least one absorption band that does not overlap with others and allows the compound to be unambiguously isolated from a mixture of intermediate or by-products at any stage of preparation. The analysis time is 25 min, the maximum random relative error is 1.2%. Table 2 shows the spectral characteristics of oligomers that are the main products of OTFE reactions with acid fluorides in aprotic solvents of diglymes-type (as impurities can be the products of OTFE interaction with a solvent – i. e. corresponding alcohol and simple ethers) [15]. These oligomers, as substances containing a reactive fluorine hydride group, can be converted into various derivatives: acids, esters, amides, alcohols, nitriles, etc. CF3O(CF2CF2O) fragment, common for all compounds, has spectral signals in the same region as oligomer 2, however, the presence of its own characteristic band for each compound makes it possible to analyze their mixtures in any component ratio. It is just this task that arises when monitoring the completeness of reactions course for obtaining a particular compound, i. e. it is necessary to be able to analyze a reaction mixture containing, as a rule, several components at once. So, for example, when monitoring the production of esters 12, the reaction mixture at various stages may contain the initial oligomer 3, its impurities 5-7, 9 and the final ester 12. Compounds 3, 6 and 7 have close chemical shifts, but practice has shown that regularities in chemical shifts δ (CF2COF) 76 ppm <δ (CF2COOCH3) 77 ppm <δ (CF2COOH) 78 ppm observed quite strictly.
Table 1. Spectral characteristics of tetrafluoroethylene oxide oligomers and their derivatives.
Perfluoropolyoxoalkylvinyl ethers
General scheme for preparation of perfluoropolyoxoalkylvinyl ethers is as follows [16]:
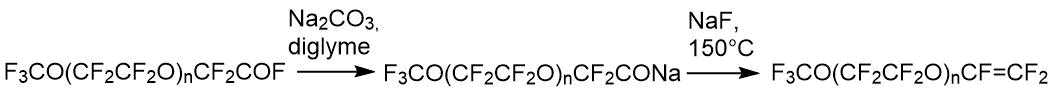
The given chain of reactions was analyzed by NMR at all stages - from initial to final products. CF3O(CF2CF2O)n-fragment, common for all compounds, is characterized in 19F spectrum by absorption lines in following in spectral regions - triplet -55 ÷ -57 ppm. (CF3O-), pre-end quartet -89 ÷ -90 ppm (CF3OCF2-) and multiplet of internal fragment 87 ppm (-CF2CF2O-)n. In addition to absorption lines listed above, each compound has its own characteristic lines in 19F spectrum. Thus, oligomer 1 has a spectral signal at -76 ppm corresponding to – CF2COF, compound 2 is characterized by a specific absorption band at –129 ppm (-CF(CF3)-), and final ester 3 that is characteristic for three fluorines with double bond is a set of absorption lines at -115, -122, -135 ppm. The presence of such characteristic non-overlapping signals makes it possible qualitative (and when comparing the integral intensities of the signals) and so quantitative unambiguously identify the composition of both the main and intermediate products of given reactions. The presence of hydrogen-containing impurities and water in soda or diglyme under our production conditions led to formation of such by-products as hydrides with general formula of F3CO(CF2CF2)nCFHCF3. Their presence is monitored using PMR spectroscopy, in which the hydride gives a doublet of quartets (JH-F = 51.8 Hz, J H-CF3 = 2.7 Hz) and absorption band at 6.05 ppm. In 19F spectrum, the characteristic absorption band corresponding to -CHF- group is in the spectral region of -145 ppm, thus formation of such hydride can be monitored both qualitatively and quantitatively.
Hydrides can be used as separating fluids for submersible pumps. In addition to hydrides with general formula 4, CF3O(CF2CF2O)nCF2H hydrides were synthesized and analyzed. 19F spectrum of such hydrides has characteristic line – doublet of triplets in spectral region of -84.3 ppm. (JCF2-H=67.0 Hz, J CF2-F=6.0 Hz), in PMR spectrum - triplet in spectral region of 6.45 ppm. (JH-CF2≈67.0 Hz) respectively.
Perfluoropolyether carboxylic acids and their methyl esters
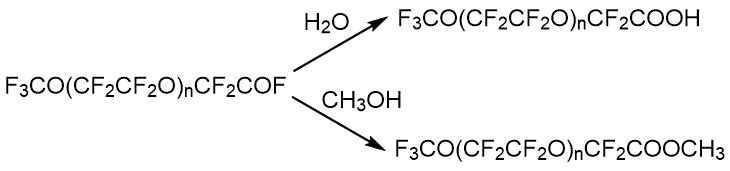
Analysis of acids CF3O(CF2CF2O)nCF2COOH with n = 1-4 based on tetrafluoroethylene oxide oligomers by 19F NMR is not particularly difficult. For any n, acids have its own characteristic line in spectral region of 78.4 ppm. Its methyl ester and residues of free and bound diglyme, which were not previously described, were found as impurities in the acid samples [17]. Formation of ether 7 proceeds from the ether with general formula CF3O(CF2CF2O)n-1CH3 presented in the starting oligomer. Its formation was confirmed by a special experiment, including various treatments of obtained acid and NMR analysis of starting oligomer and acid after treatment (1H, CF2OCH3 3.76 ppm). On average, the starting oligomer contained 8% mol. of ether 8. When perfluoroacids 5 were prepared in an acidic medium, -CF2CF2OCH3 was transformed into -CF2COOCH3 with disappearance from spectrum the spectral line at -94 ppm, and with appearance of signal for substituted methylcarboxyl group at 3.70 ppm. Fluorination under harsh conditions does not give an effect, the content OCH3 remains at the level of 8%. Diglyme can be completely removed by treatment with sulfuric acid, leaving about 5% of the esters.
Another commercial product is methyl esters of perfluoropolyether carboxylic acid. The spectra of acid methyl ester samples clearly show the presence of ether, which is obtained during production of initial oligomer (due to interaction with diglyme). Analysis of various fractions of distillation of methyl ester showed that at 111°С the mixture contains the maximum amount of ether and such impurity as diglyme, and at 130 °С the spectral line of ethers disappears.
Alcohols based on perfluoropolyether carboxylic acids
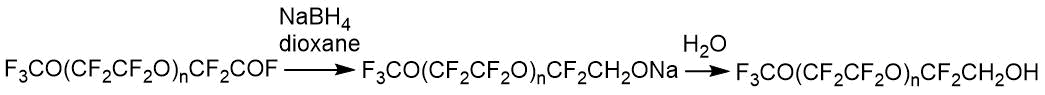
The target alcohols can be used to produce acrylates. Alcohols with n=1-5 were analyzed by 19F NMR.
Table 2. NMR chemical shifts for main groups of analyzed alcohols.
|
Group |
Chemical shift, ppm |
|
-CH2- |
1H: 3.73 t |
|
-OH |
1H: 4.7 (maybe interactions with impurities) |
|
-(CF2CF2O)n- |
19F: -88,2 |
|
-CF2CH2- |
19F: -80,6 |
|
CF3O- |
19F: -56,3 |
|
CF3OCF2O- |
19F: -90,2 |
In the presence of water at first stage, the corresponding acids can be formed, which is fixed in the spectral region of -77.6 ppm. (-CF2COOH). An uncharacteristic position of signal for –OH group may also indicate an acidic medium in mixture under study. Among the products of subsequent hydrolysis, formation of trifluoroacetic acid with characteristic chemical shift of -75.6 ppm is noted. Herewith, in some experiments, its amount reached 12% mol.
Polyoxymethylenes

In series of organofluorine compounds, the oxygen-containing derivatives with structure of polyethers I and II, which are obtained by oxidation of tetrafluoroethylene by molecular oxygen in the presence of a chemical initiator, have become widespread.
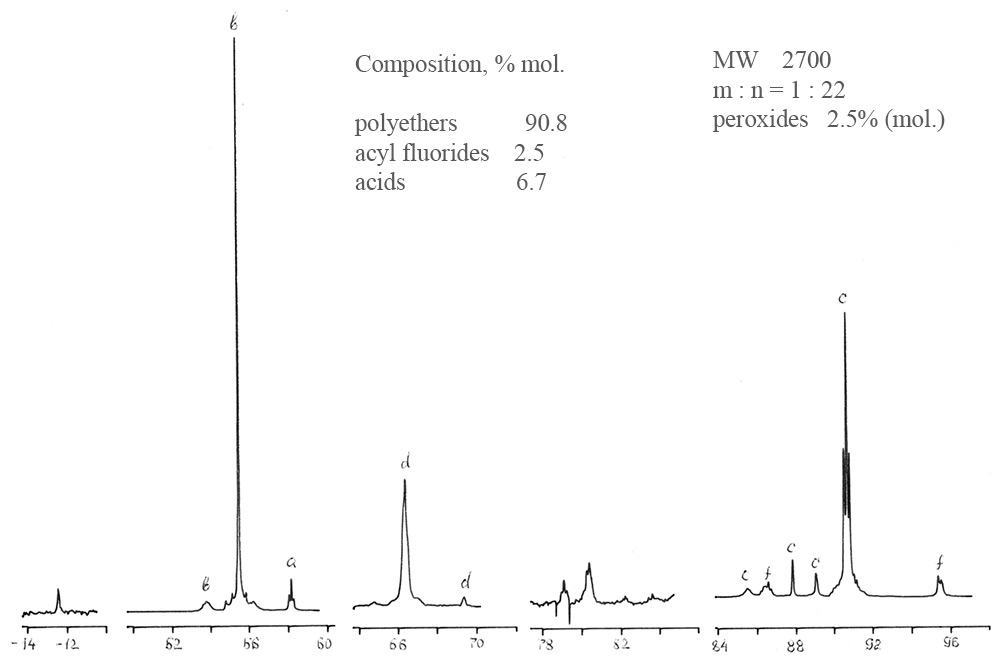
Figure 1. 19F NMR spectrum of crude polyether I.
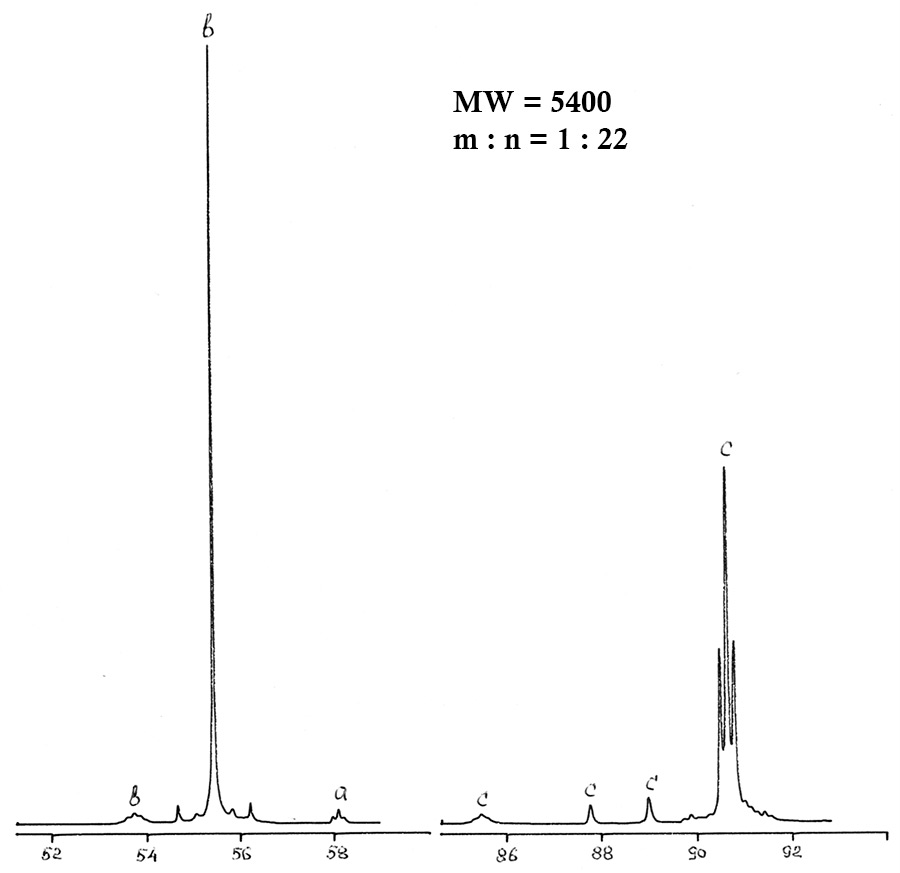
Figure 2. 19F NMR spectrum of polyethers II after treatment.
Real practice has shown that traditional methods of analysis - chromatography, IR spectroscopy, chemical analysis - do not provide exhaustive information about polyethers, do not allow to determinate their fragmented composition and monitoring of synthesis. 19F NMR analysis allows to determinate the fragment composition, average molecular weight and percentage of components containing peroxide (active) oxygen. The total relative error is 7% in the range of measurement of molecular weight and in the range of density from 102 to 104 Da (amu). The assignment of spectral lines for polyethers II type CF3O(CF2O)m(O)x(CF2CF2O)n(O)yCF3 is given in Table 3.
Table 3. 19F chemical shifts for main groups of analyzed polyethers.
|
Group |
19F chemical shift, ppm |
Spectrum designation |
|
-CF2O- |
-52,7÷-54,4 |
b |
|
-CF2CF2O-O- |
-85,2÷-94,4 |
f |
|
-CF2O-O- |
-64,2÷-66,2 |
d |
|
CF3O- |
-57,2 |
a |
|
CF3CF2O- |
-86,0÷-89,6 |
c |
Polyoxymethylene (POM) production technology includes the production of crude with molecular weight of 2400-3400 Da in the freon-318 medium, with solvent stripping, thermal stabilization and (in some cases) – with chemical treatment to reduce the amount of peroxide and acid fluorides and to increase the stability and inertness of target polyethers. During synthesis of polyethers I, are formed next functional compounds – acyl fluorides and acids, which are converted into "closed" structures by treatment by fluorine. The completeness of this treatment is controlled by disappearance of spectral lines in the range from -79 ÷ -80 to +12.4 ppm. Fluorine treatment also allows one to get rid of other hydrogen-containing impurities, the presence of which is qualitatively recorded in PMR spectrum.
NMR spectroscopy was used to study the dependence of molecular weight on the amount of worn-out monomer. It was found that dependence in above-noted interval is linear.
Conclusion
Thus, analysis based on 19F NMR method, supplemented by PMR spectroscopy (taking into account its operability and informativity), makes it possible to control the process of obtaining various polyethers and their derivatives, control of yield of target products and also - quickly optimize the technology.
References
- Pospelova N. B., Mokrushin I. G., Specific feature of NMR analysis of perfluorinated compounds. Bulletin of Perm University, Series: Chemistry, 2016, 3(23) 85-91. (in Russian)
- Pospelova N. B., Mokrushin I. G., Identification method of hexafluoroprylene dimers and trimers by 19F NMR. Fluorine notes, 2017, 115, 5-6.
- Emsley J. W., Feeney J., Sutcliffe L. H. High resolution nuclear magnetic resonance spectroscopy. Elsevier, 1966, Т. 2.
- Cavanaugh R., Atkins G. Epoxidation of tetrafluoroethylene and chlorotrifluoroethylene. Patent US 3775440, 1973.
- Ginsburg,V.A. et al., Doklady Chemistry, 1963, 149(1), 188. (Doklady Akademii Nauk SSSR, 1963, 149(1), 97).
- Soshin V. A. et al., Method of producing tetrafluoroethylene oxide, Patent RU 1840802, 2010.
- Mukhametshin F. M. et al., Tetrafluoroethylene oxide production process, Patent SU 1840594 2007.
- Maksimov, B. N. Russian Journal of Organic Chemistry, 1994, 30(12), 1935.
- Mezentsev, A. I.; Poluektov, V. A. Russian Journal of Physical Chemistry, 1983, 57(7), 1014. (Zhurnal Fizicheskoi Khimii, 1983, 57(7), 1672).
- Agopovich, John W.; Gillies, Charles W. Journal of the American Chemical Society, 1980, 102(25), 7572.
- Agopovich, John W.; Gillies, Charles W. Journal of the American Chemical Society, 1983, 105(15), 5047.
- Czarnowski, Joanna. Journal of Fluorine Chemistry, 2008, 129(4), 261.
- Sianezi, D., Marrachchini, А., Marchionni, D, Method for production of peroxidized perfluoropolyethers. Patent RU 2070558, 1997.
- Maksimov B. N., Barabanov V. G., Serushkin I. L., Industrial fluoroorganic products, Handbook, 1996.
- Leo W. J. Fluorocarbon ethers of tetrafluoroethylene epoxide. Patent US 3250806, 1966.
- Mukhametshin F. M. et al., Method for preparing perfluoropolyethers , Patent SU 18405618 2007.
- Ponomarenko V. A., Krukovsky S. P., Alybina A. Yu., Fluorine-Containing Chain Polymers, Moscow, 1973, 56-80. (in Russian)
ARTICLE INFO
Received 11 August 2020
Accepted 18 August 2020
Available online October 2020
Recommended for publication by Prof. S.M. Igumnov
Fluorine Notes, 2020, 132, 1-2
