Received: July 2020
DOI 10.17677/fn20714807.2020.04.02
Fluorine Notes, 2020, 131, 3-4
1,1'-DI[METHACRYLOYLOXY-BIS(TRIFLUOROMETHYL)METHYL] FERROCENE: TWO IN ONE – ANTIOXIDANT AND CROSSLINKER
O. A. Melnik, V. I. Dyachenko
A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Vavilov St. 28, 119991, GSP-1, Moscow, Russian Federation
e-mail: omel@ineos.ac.ru
Abstract: Copolymers of methyl methacrylate and 1,1'-di[methacryloyloxy-bis(trifluoromethyl) methyl] ferrocene were synthesized, their structure, solubility and thermal characteristics were studied. It has been established that introduction of 1-3 mol.% of ferrocene-fluorine-containing monomer into poly(methyl methacrylate) chain leads to a significant increase in its thermal and thermo-oxidative stability.
Keywords: 1,1'-di[methacryloyloxy-bis(trifluoromethyl)methyl] ferrocene, poly(methyl methacrylate), free-radical copolymerization, heat resistance
The service life extension of polymeric materials, as well as expanding the temperature range of their use in various technical apparatus and devices, is of great practical importance. Traditionally, this problem is solved by introducing into polymer the antioxidant additives, which form a mechanical mixture with this polymer. It is known to use the ferrocene and its derivatives as such additive and as one of the most accessible organometallic compounds with a low oxidation potential [1-3]. The ferrocene-containing polymers are used to obtain some organic polyelectrolytes [4], liquid crystals [5] and composite materials [6].
Copolymerization of metal-containing compounds with traditional vinyl monomers makes it possible to modify known polymers in order to increase their functional (including thermal) characteristics [7]. As a rule, the copolymerization reaction proceeds in accordance with a free-radical mechanism, since the corresponding processes are of great practical importance and are easier to study. Thus, it was shown that the introduction of additive (vinyl- or acetylenyl ferrocene) in amount of 10–15% during polymerization (for example, of styrene or isoprene) makes it possible to significantly increase the temperature of thermal destruction of corresponding polymers [8].
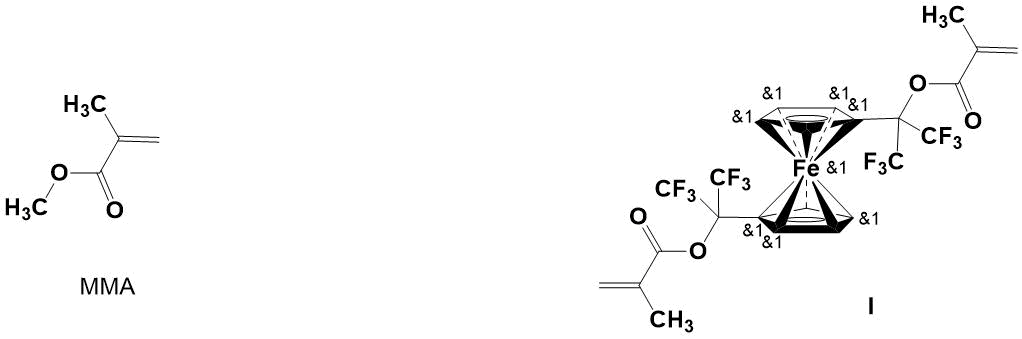
Earlier, the authors reported on synthesis of 1-trifluoromethyl-1-ferrocenyl-2,2,2-trifluoroethyl methacrylate (TFMA) [9] and “side-chain” homo- and copolymers based on it [10, 11]. The presence of two CF3 groups and a ferrocenyl substituent in the ester part of its molecule made it possible to obtain α,α-bis(trifluoromethyl)ferrocenyl-containing polymers and copolymers with methyl methacrylate (MMA), soluble in organic solvents. It has been shown that introduction of 5 mol. % TFMA into polymer chain of poly(methyl methacrylate) (PMMA) results in an increase in its thermal and thermo-oxidative stability by 15°C and 55°C, respectively. At the same time, MMA polymer with addition of 5 mol. % ferrocene (with its mechanical mixing) is inferior in thermal resistance to copolymers in which ferrocenyl groups (acting as an antioxidant) are attached to polymer chain via a covalent bond (see Fig. 1).
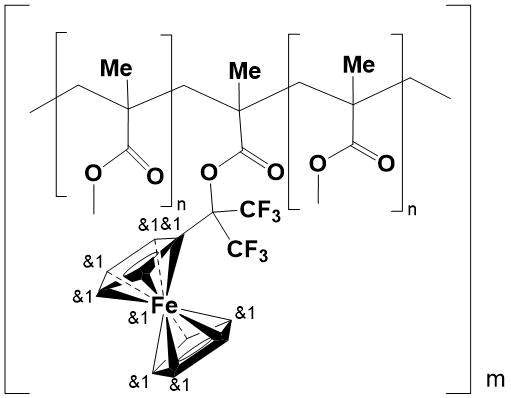
Figure 1.
Such advantages of spatially reticulated polymers as high thermal stability, resistance to solvents and good mechanical properties are widely known [12]. To impart the new practically important properties to known polymers, it seemed appropriate to study the possibility of using bifunctional ferrocene-containing compounds in a synthesis of network polymer systems.
In this paper, the copolymers of MMA and 1,1'-di[methacryloyloxy-bis(trifluoromethyl)methyl] ferrocene (I) with various compositions were synthesized by free-radical copolymerization in bulk, and their thermal characteristics were studied.
All copolymers are transparent, solid, glassy yellow specimens, insoluble in organic solvents, alcohols and water. Their structure has been confirmed by elemental analysis and IR spectroscopy. In this case, network copolymers are formed as a result of in situ introduction into polymer chain of resulting PMMA 1,1'-bis(1,1,1,3,3,3-hexafluoroisoprop-2-yl) ferrocene-containing component, which acts as a crosslinker (Fig. 2).
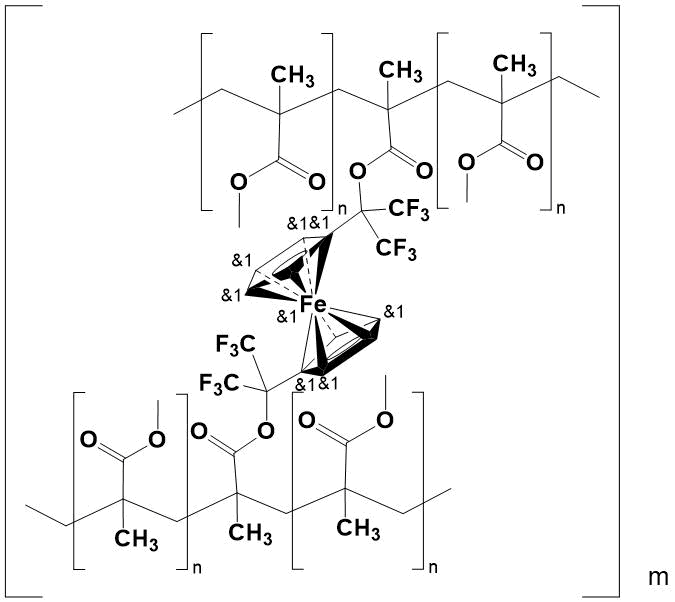
Figure 2.
Due to crosslinking, the inner pore surface decreases. This makes difficult of diffusion and reduces the aerial oxygen content in the copolymer, that capable of causing the destruction of polymer chains under extreme conditions. In turn, the low ionization potential of Fe2+ ion in Fe3+ in ferrocenyl core, as well as the reversibility of this process without violating the geometry of the molecule [1], also encourage to protection of copolymer from oxidation.
Thermal stability of obtained copolymers was estimated by onset temperature Td, at which the weight loss of analyzed sample was 10% of initial one. It was determined by dynamic thermogravimetric analysis at heating rate of 10°C/min in air and in argon atmosphere. The results are shown in Table1.
It was found that Td of copolymer MMA and compound I, containing 1 mol. % of I-component, is 320 °C in air and 360°C in argon. For copolymer, containing 3 mol. % of I-component, Td is 330°C in air and 350°C in argon.
Comparison of obtained results with the data of thermogravimetric analysis of MMA homopolymer and copolymers MMA with the monomethacrylate derivative of ferrocene TFMA indicates a significant improvement in thermal stability, especially under oxidizing conditions (in air), after introduction into MMA the dimethacrylate derivative of ferrocene I as a comonomer (see Table 1). So, for example, Td in air for PMMA is 265°C, for MMA copolymer, containing TFMA with a molar composition 97:3 is 270°C, and for MMA copolymer, containing dimethacrylate I with a molar composition 97:3 is 330°C. The thermal stability of MMA copolymers, containing dimethacrylate I, exceeds the thermal stability of PMMA by 55-65°C in air, and by 30-40°C in argon. With the same comonomer content in air, the onset temperature of MMA copolymers, containing TFMA is 45-60°C lower than Td of MMA copolymers, containing dimethacrylate I.
Table 1. Thermal characteristics of PMMA and MMA bulk copolymers, containing TFMA and compound I
|
Thermal characteristics of (co)polymer |
PMMA |
PMMA with additive (5 mol.% of ferrocene) |
Copolymer MMA/TFMA, mol.% * |
Copolymer ММА/compound I, mol.% |
|||
|
99:1 |
97:3 |
95:5 |
99:1 |
97:3 |
|||
|
Тd in air |
265 |
285 |
275 |
270 |
320 |
320 |
330 |
|
Тd in argon |
320 |
300 |
330 |
325 |
335 |
360 |
350 |
*according to [11]
Thus, the use of ferrocene-fluorine-containing dimethacrylate I, which is both аn antioxidant and а crosslinker of polymer chains, leads to an increase in thermal resistance and thermo-oxidative stability of PMMA by 30-40°C and 55-65°C, respectively. Apparently, compound I can be used in a similar way to improve the heat resistance of other vinyl polymers.
Experimental part
1H and 19F NMR spectra were recorded in CDCl3 on a Bruker Avance 400 instrument (400,13 and 382 MHz, respectively). Chemical shifts 1H are given relative to Me4Si (internal standard), and 19F - relative to CF3CO2H (external standard). IR spectra were recorded via Nicolet Magna-750 spectrophotometer. Mass spectra were recorded on a Finnigan MAT INCOS 50 quadrupole mass spectrometer (with direct input, ionization energy 70 e-V). Thermogravimetric analysis was carried out using a MOM Q-1500 derivatograph.
1,1'-Di[methacryloyloxy-bis(trifluoromethyl)methyl]ferrocene (I) was synthesized as described in [13]. Melting point: 45-46оС (petroleum ether).
Founded,%: C, 44,33; H, 2,71; F, 34,78. C24H18F12FeO4.
Calculated,%: C, 44,06; H, 2,77; F, 34,85.
1H NMR (CDCl3, δ, ppm): 6,19 (br s, 1H, CH2); 5,73 (br s, 1H, CH2); 4.49 (br s, 8H, 2C5H4); 1.95 (br s, 6H, 2CH3).
19F NMR(CDCl3, δ, ppm): -6,28 (s).
Mass spectrum, m/z (I,%): 654 [M] + (100), 586 (34), 570 (10), 226 (27), 195 (19), 69 (7).
Preparation of copolymer MMA and dimethacrylate I at a molar ratio of components 99:1
To a mixture of 2,48 g of freshly distilled MMA (Aldrich, 99%) and 0,16 g of 1,1'-di[methacryloyloxy-bis(trifluoromethyl)methyl] ferrocene (I) was added 0,013 g (0,5 wt. %) of dinitrile of azobisisobutyric acid as a copolymerization initiator. The reaction mixture prepared in this way was filtered into a glass ampoule, that was degassed by freezing while immersed in liquid nitrogen, followed by thawing in a vacuum. This procedure was repeated 3 times. After that, this ampoule was sealed off and placed in a thermostat heated to 60°C. After 4 hours, the ampoule was removed, cooled and opened. Transparent yellow solid copolymer was dried under vacuum at 40 °C for 24 hours to constant weight.
Founded,%: C, 59,19; H, 7,68; F, 2,21.
Calculated,%: C, 59,06; H, 7,75; F, 2,16.
The IR spectrum of copolymer contains absorption bands characteristic of both MMA component and compound I: 3010, 985, 965, 839, 483 cm–1 (the ferrocene fragments); 1192 and 1140 cm–1 (CF3); 1724 cm–1 (C=O of methyl methacrylate) and 1745–1 (C=O of monomer I). There are no absorption bands of stretching vibrations of C=C bonds at 1634 and 1645 cm–1, which were present in the IR spectra of initial monomers.
MMA copolymers with dimethacrylate I were synthesized in a similar way at a molar ratio of components 97:3.
Acknowledgments
The authors are grateful to M. I. Buzin for carrying out the dynamic thermogravimetric analysis. This work was performed with the financial support from Ministry of Science and higher Education of the Russian Federation using the equipment of Center for molecular composition studies of INEOS RAS.
References
- Nesmeyanov А. N., Ferrocene and related compounds, Moscow: Nauka, 1982, 439 pр.
- Perevalova E. G., Reshetova M. D., Grandberg K. I., Methods of organoelement chemistry. Iron organic compounds, Ferrocene, M., Nauka, 1983.
- Encyclopedia of Polymer Science and Engineering, N-Y, Wiley-Interscience, 1987, 10, 541 pp.
- Gao Y., Shreeve J. M., Journal of Polymer Science, Part A: Polymer Chemistry, 2005, 43, 5, 974-983.
- Senthil S., Kannan P., Journal of Polymer Science Part A: Polymer Chemistry, 2001, 39, 14, 2396-2403.
- Igumnov S. M., Dyachenko V. I., Melnik O. A., Sokolov V. I., Nikitin L. N., Fluorine-containing monomers, (co)polymers and composite pyrocarbon materials based on them. Chapter 11 in the book Fluoropolymer materials, ed. V. M. Buznik, NTL Publishing House, Tomsk, 2017, 472-503.
- Pomogailo A. D., Savostyanov V. S., Metal-containing monomers and polymers of their basis., Moscow: Khimiya, 1988, 384 pp.
- Lemenovsky D. A., Sandwich metal complex compounds, Ferrocene, Soros Educational Journal, 1997, 2, 64-69.
- Dyachenko V. I., Nikitin L. N., Melnik O. A., Peregudova S. M., Peregudov A. S., Igumnov S. M., Khokhlov A. R., Fluorine notes, 2011, 79(6).
- Melnik O. A., Dyachenko V. I., Nikitin L. N., Blagodatskikh I. V., Buzin M. I., Peregudova S. M., Vygodsky Ya. S., Igumnov S. M., Khokhlov A. R., Doklady Chemistry, 2012, 443(6), 692-695.
- Melnik O. A., Dyachenko V. I., Nikitin L. N., Blagodatskikh I. V., Buzin M. I., Yurkov G. Yu., Vygodsky Ya. S., Igumnov S. M., Buznik V. M., Polymer Science, Series A, 2013, 55(11), 1315‑1320.
- Kireev V. V., High molecular weight compounds, M., Higher school, 1992.
- Dyachenko V. I., Melnik O. A., Nikitin L. N., Igumnov S. M., Fluorine notes, 2018, 117(2).
ARTICLE INFO
Received 13 July 2020
Accepted 15 July 2020
Available online August 2020
Recommended for publication by PhD V. Don
Fluorine Notes, 2020, 131, 3-4
