Received: October 2019
DOI 10.17677/fn20714807.2019.06.03
Fluorine Notes, 2019, 127, 5-6
HYDROPHOBIC PROPERTIES STUDY OF FLUORINE-CONTAINING ULTRA-THIN COATINGS OF POLYESTER MATERIALS OBTAINED IN THE SUPERCRITICAL CARBON DIOXIDE
A.A. Pestrikova1 , E.D. Gorbatyuk2 , A.Yu. Nikolaev1 , V.I. Dyachenko1 , I.S. Chashchin1 , O.A. Serenko1 , S.M. Igumnov1
1A.N.Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences, 119991, GSP-1, Moscow, V-334, Vavilova St. 28.
2AESC MSU, 11, Kremenchugskaya str., Moscow, Russian Federation, 121357.
e-mail : pestrikova@ines.ac.ru
Abstract: The water-repellent properties of newly synthesized hydrophobizing agents of a three-module molecular type structure 4a, b, deposited on polyester and polycotton fabric using SC-CO2 technology was studied. It is shown that in vast cases the contact angle (θ) of hydrophobized surfaces exceeds 140°. An additional temperature treatment of investigated samples was applied at 120°C which makes it possible, in the case of activated cotton-polyester fabric, to increase θ to 148°.
Keywords: hydrophobicity, supercritical carbon dioxide, polyethylene terephthalate, fluorine-containing water repellents.
The hydrophobization of the surface of materials is one of the effective ways to extend their operational lifetime [1]. To solve this problem, as a rule, utilize the application of ultrathin as well as monomolecular layers of fluorine-containing or other organic and inorganic compounds on their surface [2]. However, to achieve superhydrophobicity of coatings (θ ≥ 150°), in addition, the micro- and nanoscale multimodality of coating surface is required [3]. Not less important task is to fix the hydrophobizing agent on the surface of metal, fabric or other material. Usually, in these cases, chemical agents are utilized containing molecules (in addition to hydrophobic spacer) of anchor group (AlkO)*3Si (or other group) capable of chemically bonding to the surface of a material [4]. It is the combination of these conditions that allows us to hope for successful creation of new effective hydrophobic coatings.
The present work is devoted to study of water-repellent properties of our recently synthesized new hydrophobisators of the three-module type of the structure of molecule N-[3-triethoxysilyl) propyl] -3- (2,2,3,3,4,4,5,5,6,6, 7,7-dodecafluoroheptyloxy) propane-1-sulfamide (4a) and N- [3-triethoxysilyl) propyl] -3- (2,2,3,3,4,4,5,5,6,6, 7,7,7-tridecafluoroheptyloxy) propane-1-sulfamide (4b), starting from commercially available polyfluorinated alcohols (1), propansultone (2) and 3-triethoxysilylpropylamine (3) (see Fig. 1) [5].
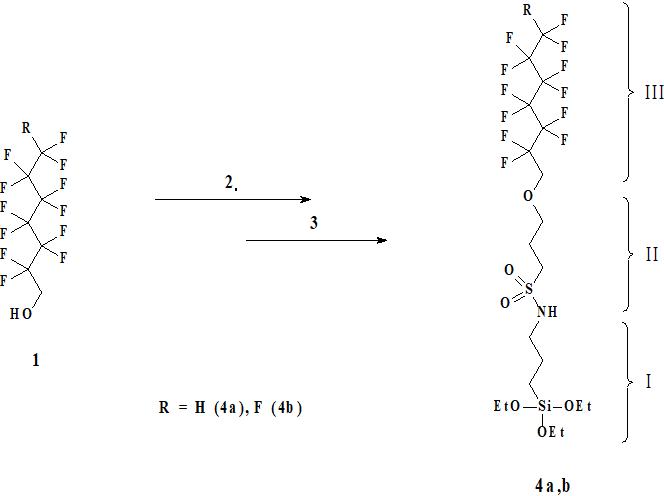
Figure 1. The scheme of 4a,b structures` synthesis.
The molecule of compounds 4a,b consists of an anchor group (EtO),Si included into N-(3-triethoxysilylpropyl)amide component (I), a connecting unit (II) formed by opening of the 1,3-propanesultone cycle, and a functional hydrophobic spacer - polyfluoroheptyloxy group (III) (see Fig. 1).
To eliminate potential coating defects that arise when applying a hydrophobisator by precipitation from a solution or during spraying, we utilized supercritical carbon dioxide (SC-CO2) as a solvent. It is known that it does not possess surface tension due to which it can transfer the substances dissolved in it to places inaccessible to their solutions in traditional solvents. In addition, its solvent capacity can be controlled by changing the temperature and pressure of the process [6].
On the other hand, polyfluorine-containing compounds, unlike non-fluorinated ones, have the ability to dissolve in SC-CO2.
Thus, having studied the solubility of 4a,b in SC-CO2, they were deposited onto fabric samples under the same conditions. Namely, the application of compounds 4a,b on the fabric was carried out in SC-CO2 at a pressure of 400 atm., a temperature of 65 oC and stirring for 4 hours. The process was carried out using the installation depicted in Fig. 2 [7].
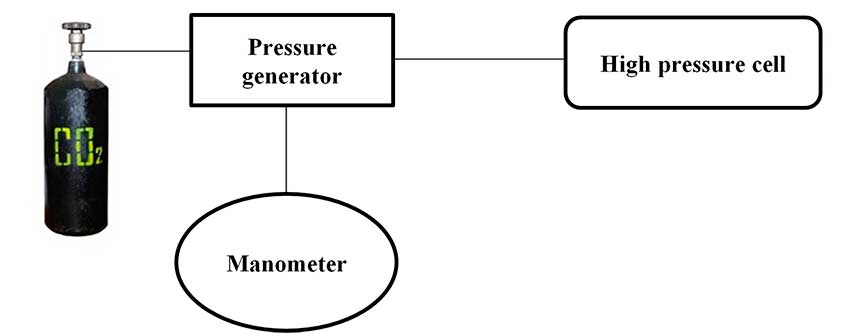
Figure 2. Installation for applying substances 4a, b to the fabric in SK-CO2.
After applying the compounds, the cell was cooled to room temperature, the pressure in it was brought to atmospheric pressure, the cell was opened and fabric was removed.
As a result, samples of activated [8] and non-activated polyester (PE) and polycotton (PCot) fabrics with compounds 4a,b applied on them were obtained. After that, post-processing of samples in water vapor at 100 °C was carried out for 1 hour. The samples dried under normal conditions to a constant weight were examined for their hydrophobic properties by measuring the contact angle, as well as the structural features of coatings. their
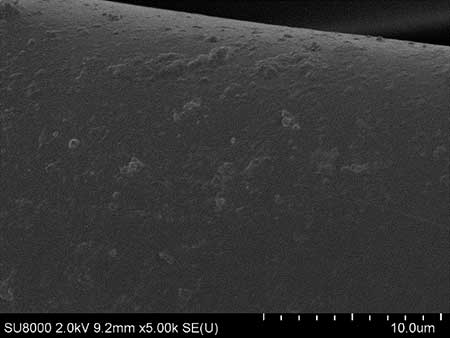
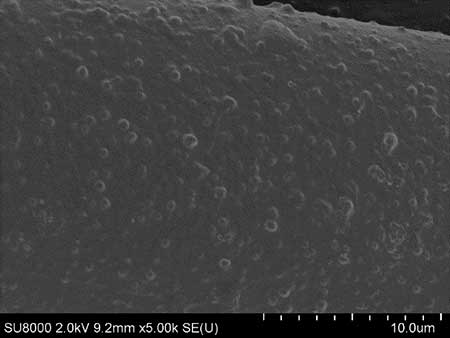
The microstructure of resulting coatings was analyzed using scanning electron microscopy (SEM). The micrographs (see Fig. 3a,b) of the hydrophobic coating of cotton and cotton polyester fabrics indicate the uniformity and proportionality of the coating with fluorine-containing compounds 4a,b. On the microphoto (see Fig. 3b) it is seen that during deposition of 4b on the substrate, tubercles of regular shape are formed, the diameter of the base of which does not exceed 1 micron.
|
|
|
Figure 3. SEM micrographs of the coatings of activated polycotton (PCot-A) tissue obtained using water repellents 4a (a) and 4b (b).
This type of surface modality is characteristic for coatings applied using SC-CO2. Only a combination of modality and coating hydrophobicity can provide high contact angle values [1].
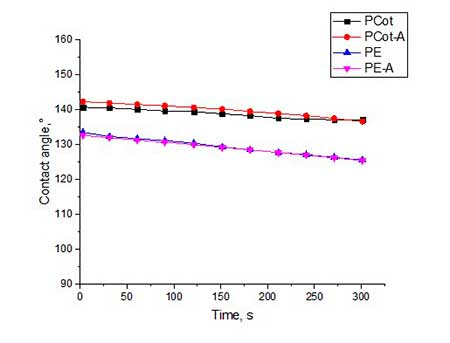
Fig. 4a and Fig. 5a show the results of contact angles analysis for coatings obtained by hydrophobizing agents 4a,b using post-treatment in the form of moist curing. Such procedure promotes the hydrolysis of Si-OEt bonds and the subsequent polycondensation of fluoroalkyl-containing siloxanes 4a,b. On the other hand, cellulose, which is part of the polycotton fabric, also has numerous -OH groups which a fluorine-containing siloxane scaffold is attached due to the formation of a Si — O-cellulose bond.
|
|
|
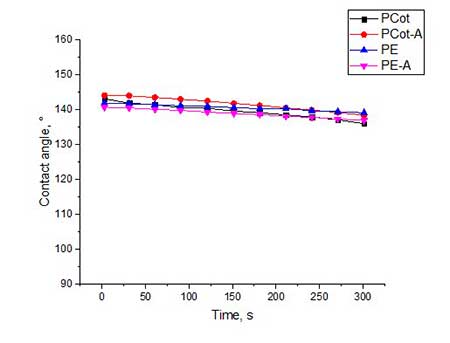
Figure 4. The contact angles for all types of fabrics, treated by hydrophobisator 4а and by post-treatment in water vapor (4a) and by subsequent heat treatment at 120 oC (4b).
|
|
|
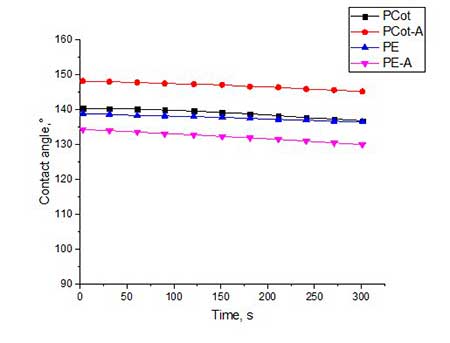
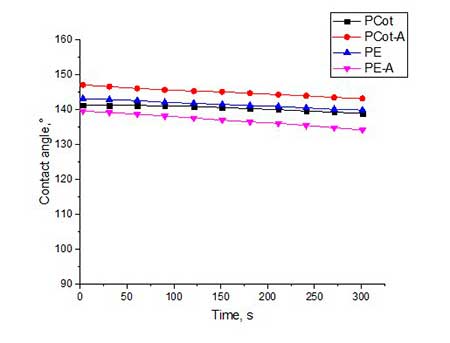
Figure 5. The contact angles for all types of fabrics, treated by hydrophobisator 4b and by post-treatment in water vapor (5a) and by subsequent heat treatment at 120 oC (5b).
From graphical data it can be seen that the contact angles for all types of fabrics treated with compound 4a is 133-142 o (see Fig. 4a). The slightly higher values of θ (140-144 о) for fabric samples treated with 4b having at the end of polyfluorinated spacer are not CF2H-, and the -CF3 group (see Fig.5a).
Trying to increase the value of contact angle, we utilized additional heat treatment of all samples of polyester and polycotton fabrics at 120 °C for 4 hours. Indeed, for some fabric samples the θ value increased by 3-6 °. Most of all, this was manifested on activated samples of polycotton fabric. So, for PCot-A fabric treated with hydrophobisator 4b and after additional heat treatment, θ increased from 144o to 147o, and in the case of the same fabric, but treated with hydrophobisator 4a, θ increased from 142o to 148o. Apparently, during heat treatment, a partial rearrangement of siloxane framework occurs which causes reorientation of fluorine-containing spacers in one direction and, thereby, leads to increase in coating hydrophobicity.
Experimental part
Fabric hydrophobization was carried out in SC-СО2 with purity 99.995% (according GOST No. 8050-85) produced by JSC MGPZ. For hydrophobization, we used polyester (PE) and polycotton fabric (PCot) with a surface density of 180 ± 10 g/m2 and the number of threads 216 ± 4 per 10 cm warpwise and 203 ± 4 per 10 cm weftwise [9], and also activated PE and PCot fabrics according to the method described in [8] - polyester (PE-A) and activated cotton-polyester (PCot-A) fabric, respectively.
The application of hydrophobisators 4a,b onto fabrics was carried out on the apparatus (see Fig. 1) at: pressure 400 Atm and temperature of 65 °C for 4 hours according to method described in [7]. Fabric post-processing was carried out in water vapor at 100 °C for 1 hour. Additional heat treatment of fabrics was carried out at 120 °C for 4 hours. The contact angle was determined by sessile drop method on instalation KRUSS EasyDrop (Germany). Micrographs of coatings were obtained using scanning electron microscopy Carl Zeiss Supra 40.
General procedure for applying hydrophobisators 4a,b onto PE, PCot, PE-A and PCot-A fabrics
0,2 g of Hydrophobisator was placed in 20 ml steel cylindrical high-pressure cell. This cell was divided into two parts (upper and lower) with metal mesh of non-magnetic material, where the element of magnetic stirrer is located. 1 cm2 of fabric was placed on metal mesh, the cell was tightly closed and connected to a system with carbon dioxide (Fig. 2). After CO2 was supplied, the pressure in the cell was brought up to 400 Atm using a gas press (Thar, USA) and sealed. The cell was then disconnected from the CO2 supply system and immersed in a water bath equipped with magnetic stirrer. The stirrer was turned on, the temperature of water bath was raised to 65 °C, and the dissolution process of hydrophobisator was carried out for 4 hours. Then the pressure was slowly released with simultaneous precipitation of hydrophobisator onto fabric, the cell was opened and the fabric was removed. The applying of fluorine-containing compounds 4a,b on all types of fabrics was carried out under the same conditions: pressure 400 Atm., temperature of 65 °C for 4 hours.
General procedure of fabric post-processing after applying a hydrophobisator
After applying of hydrophobisator, fabric sample was fixed over a boiling water bath at a distance of 2 cm from the surface of water. This processing was carried out for 1 hour. Then the fabric was dried under normal conditions to constant weight.
The post-processing of fabric samples all types was carried out under the same conditions (see Fig. 4a and Fig. 5a).
General procedure of additional (thermal) processing of fabric
The fabric sample post-treated with water vapor, as described above, was placed into oven and kept at 120 °C for 4 hours. The sample was removed, cooled, and then the contact angle was measured. All studied tissue samples were subjected to the same additional heat treatment (see Fig. 4b and Fig. 5b).
Conclusions
- It was shown that synthesized new hydrophobisators of three-module type molecular structure 4a,b have good solubility in SC-CO2 and high hydrophobizing properties with θ ~ 140-148° demonstrated on polyester and polycotton fabrics.
- The optimal conditions for fabrics post-processing have been found combining vapor treatment with additional heat treatment at 120 °C which makes it possible to increase the contact angle by 3-6 ° in comparison with traditional processing.
- Apparently, the use of this method of temperature post-treatment may increase θ value for other already known hydrophobisators having a hydrophobic polyfluorinated spacers and an anchor group (AlkO)3Si.
Acknowledgments
This work in the field structural and analytical studies was supported by Ministry of Science and Higher Education of Russian Federation using equipment of Center for Study of molecular structure of INEOS RAS.
This work was financially supported by Russian Foundation for Basic Research (project 16-29-05334).
The authors are grateful to Dr. T. Yu. Kumeeva and Prof. N.P. Prorokova from ISC RAS for provided fabric samples.
Literature
- L.B. Boinovich, A.M. Emelyanenko. Hydrophobic materials and coatings: principles of design, properties and applications // Russian Chemical Reviews, 2008, 77(7), 583-600.
- E.E. Said-Galiev, L.N. Nikitin, A.Y. Nikolaev, Y.E. Vopilov, I.A. Garbuzova, A.R. Khokhlov, and V.M. Buznik, Fractionation of ultradisperse polytetrafluoroethylene in supercritical carbon dioxide and the chemical structures of the fractions // Polymer Science, Series A, 2015, 57(3), 271‑278.
- M.O. Gallyamov, L.N. Nikitin, A.Y. Nikolaev, A.N. Obraztsov, V.M. Bouznik, and A.R. Khokhlov, Formation of superhydrophobic surfaces by the deposition of coatings from supercritical carbon dioxide // Colloid Journal, 2007, 69(4), 411–424.
- L.B. Boinovich, A.M. Emel’yanenko, A.M. Muzafarov, A.M. Myshkovskii, A.S. Pashinin, A.Yu. Tsivadze, D.I. Yarova. The development of coatings that give superhydrophobic properties to the surface of silicone rubber // Nanotechnologies in Russia, 2008, 3(9–10), 587–592.
- V.I. Dyachenko, L.N. Nikitin, S.M. Igumnov, Synthesis of new potential hydrophobic agents composed of three-module type molecules // Doklady Chemistry, 2018, 481(1), 139–144.
- L.N. Nikitin, M.O. Gallyamov, E.E. Said-Galiev, A.R. Khokhlov, V.M. Buznik. Supercritical Carbon Dioxide: A Reactive Medium for Chemical Processes Involving Fluoropolymers // Russian Journal of General Chemistry, 2009, 79(3), 578–588.
- S.Y. Tuzova, A.Y. Nikolaev, L.N. Nikitin, A.A. Pestrikova, and I.Yu. Gorbunova, Redispersible polymers are prepared in supercritical carbon dioxide // Russian Journal of Inorganic Chemistry, 2015, 60(6), 724–728.
- N.P. Prorokova, T.Yu. Kumeeva, S.M. Kuz’min, I.V. Kholodkov. Modification of Polyester Fibrous Materials with Surface Barrier Discharge for Making Them More Hydrophilic // Russian Journal of Applied Chemistry, 2016, 89(1), 147−154.
- T.Yu. Kumeeva, N.P. Prorokova. Ultrathin Hydrophobic Coatings Obtained on Polyethylene Terephthalate Materials in Supercritical Carbon Dioxide with Co-Solvents // Russian Journal of Physical Chemistry A, 2018, 92(2), 346–351.
ARTICLE INFO
Received 22 October 2019
Accepted 12 December 2019
Available online December 2019
Recommended for publication by Prof. V. Kornilov
Fluorine Notes, 2019, 127, 5-6