Received: August 2019
DOI 10.17677/fn20714807.2019.05.04
Fluorine Notes, 2019, 126, 7-8
INCREASING THE OPTICAL TRANSPARENCY OF AMORPHOUS COPOLYMERS OF PERFLUORINATED DIOXOLES AND VINYL ETHERS USING XENON DIFLUORIDE
V.I. Sokolov1,2, A.S. Akhmanov1,2, I.O. Goryachuk1, O.R. Malyshev3, S.I. Molchanova1, E.V. Polunin3, A.A. Yarosh3
1Institute of Photonic Technologies, Federal Research Center "Crystallography and Photonics", Russian Academy of Sciences, Moscow, Russia
2Federal Scientific Center “Scientific-research institute for System Research”, Russian Academy of Sciences, Moscow, Russia
3Institute of Organic Chemistry n.a. N.D. Zelinsky, Russian Academy of Sciences, Moscow, Russia
Abstract: The amorphous perfluorinated polymers are promising for the creation of integrated optical devices operating in the near infrared (IR) spectrum due to their high optical transparency in this spectral range. During the synthesis of copolymers from perfluorinated dioxoles and vinyl ethers by radical copolymerization under ultrahigh pressure (10-14 thous. atm.), side chemical reactions (for example, the opening of the dioxolane ring) can occur. Subsequent interaction of the products of these reactions with air moisture leads to formation of carboxyl substituents - (C=O)-OH in the macromolecule, and, as a result, to increase in polymer absorption coefficient. Using the copolymer of perfluoro-2-methyl-2-ethyl-1,3-dioxol and perfluoro-n-propyl vinyl ether as an example, we showed that their treatment with xenon difluoride leads to fluorodecarboxylation of -(C=O)-OH groups and increases the optical transparency of copolymer in near IR range.
Keywords: amorphous perfluorinated polymers, copolymers, fluorodecarboxylation, xenon difluoride, light guide films.
Introduction
The amorphous perfluorinated polymers possess a higher optical transparency in the near IR-spectrum than its hydrocarbon analogues [1, 2]. This is due to the fact that the replacement of light hydrogen atoms in the macromolecule by heavier fluorine atoms result to a shift of vibrational absorption bands towards longer wavelengths and, as a consequence to an appearance of “transparency spectral windows” in the range of 1–7 μm [1]. Therefore, the amorphous perfluoropolymers, such as TeflonAF (DuPont), Cytop (Asachi Glass), Hyflon AD (Solvay), are promising for creation of various integrated optical devices operating in this spectral range, in particular, in the “telecommunication” C - range wavelengths 1530 - 1565 nm [2, 3].
The synthesis of perfluorinated polymers can be carried out from the corresponding monomers without using of radical polymerization initiators under ultrahigh pressure [4]. Such synthesis may be accompanied by side reactions, for example, the copolymerization of dioxols and ethers can lead to the opening of the dioxolane ring, with formation of the group -C(= O)-F [4]. Subsequent hydrolysis of this group upon interaction with air moisture is accompanied by formation of carboxyl substituents -(C=O-H in the side chain of copolymer [4], which increases its absorption coefficient in the near IR-range. In this article it was shown that treatment with xenon difluoride leads to decrease in number of carboxyl groups in a copolymer and to increase in its optical transparency.
Fluorodecarboxylation of dioxol- and ether-based perfluoropolymers
The copolymers of (D3)1-x(E1)x (0 <x <1) perfluoro-2-methyl-2-ethyl-1,3-dioxole (D3) and perfluoro-n-propyl vinyl ether (E1) were synthesized using ultra high pressure method. The fragment of copolymer structure is shown in the insert to Fig. 1. The resulted substances are amorphous. Their intrinsic viscosity, measured using HVROC-S viscometer (RheoSense Inc.), was [η] ≈ 0.048 ml/mg. IR absorption spectra were measured using Shimadzu 8400S FTIR spectrometer. For this purpose, by centrifugation on the KBr substrates from solutions of (D3)1‑x(E1)x in perfluorodecalin were formed films 12–18 μm thick. These samples were heated at 165 °C for 12 hours to completely removing the solvent. After that, the IR transmittance T(λ) through the structure “film/substrate” was measured. The transmission spectrum of the film with copolymer (D3)0.5(E1)0.5 is shown in Fig. 1 (see Curve 1).
As can be seen from Fig. 1, the most intense absorption band of (D3)0.5(E1)0.5 copolymer, due to fundamental vibration frequency ν1 of C–F bonds, is located near 1250 cm–1 [1]. The first harmonic ν2 of this bond, according to [1], is located near 2490 cm–1. In Fig. 1 this harmonic corresponds to the strip centered at 2481 cm–1. The vibration harmonic ν1 of C–H bonds lies near 2950 cm–1 [1] and is weakly expressed (absorption band near 2944 cm–1, see Fig. 1), which indicates the presence of only a very small amounts of residual hydrogen atoms in the copolymer macromolecule.
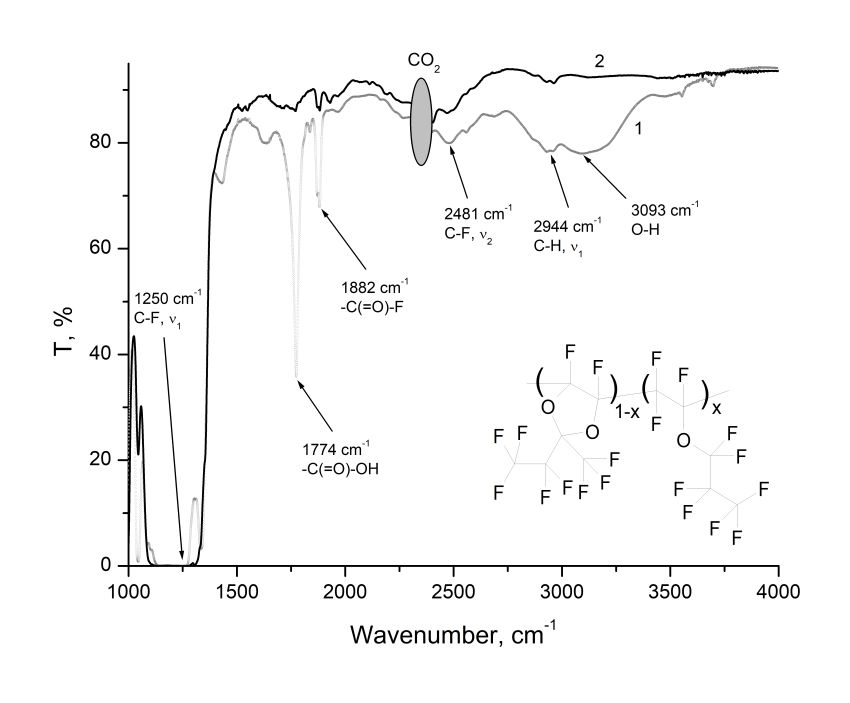
Figure 1. The transmission spectrum of the film of copolymer (D3)0.5(E1)0.5 at the KBr substrate (Curve 1).The Curve 2 is for same copolymer, but treated by XeF2. In the side insert is shown the fragment of copolymer structure, where x is the molar concentration of ether units in the macromolecule.
The absorption band centered near 3093 cm–1 is apparently due to coupled vibrational stretching of O–H bonds in carboxyl groups [5]. In addition, the spectrum contains the intense absorption lines centered at 1774 and 1882 cm–1. The spectral line at 1774 cm–1 is due to coupled vibrations of the C=O atoms in the -(C=O)-OH group, and the spectral line at 1882 cm–1 can be associated with the vibrations of these atoms in the -(C=O)-F group [5].
The presence of carboxyl groups, as well as H atoms in the copolymer macromolecule leads to decrease in its optical transparency in the near IR spectrum, including in the C range. In order to fluorodecarboxylate the copolymer, we used the xenon difluoride XeF2, which is one of the most active fluorinating agents. The Curve 2 in Fig. 1 represents the transmission spectrum of (D3)0.5(E1)0.5 copolymer treated using XeF2. This treatment was carried out as follows. The copolymer was dissolved in perfluorodecalin with concentration of 10-12 %, resulting solution was placed in a Teflon ampoule and XeF2 crystals were added there. After that, the solution was stirred on a magnetic stirrer at 65 °C for 24 hours. A copolymer film treated with xenon difluoride was formed at the KBr substrate in the same manner and under the same conditions as for copolymer film that was not subjected to fluorodecarboxylation. From a comparison of Curves 1 and 2, it follows that the XeF2 treatment leads to the disappearance of the absorption line centered near 1774 cm–1 and the band near 3093 cm–1. As a result, the optical transparency of copolymer in the near IR spectrum increased. A possible scheme for the interaction of XeF2 with carboxyl groups in the copolymer macromolecule is described by equation [6].
Rf - (C=O)-OH + XeF2 Rf - F + CO2 + Xe + HF. (1)
We believe that the process of fluorodecarboxylation using xenon difluoride is also possible for copolymers from other perfluorinated dioxoles and ethers. The copolymer (D3)0.5(E1)0.5 obtained as a result of XeF2 treatment is capable of film formation and can be used to create waveguide waveguide arrangements for integrated optics.
Conclusions
Using the amorphous perfluorinated copolymer of perfluoro-2-methyl-2-ethyl-1,3-dioxole and perfluoro-n-propyl vinyl ether as an example, it was demonstrated that its treatment using xenon difluoride leads to an increase in its optical transparency in the near IR wavelength range (due to quantity reduction of carboxyl groups in the macromolecule and increase its degree of fluorination). The resulting copolymers are capable of film formation and can be used to create various elements of integrated optical devices.
Acknowledgments
This work was supported by Ministry of Science and Higher Education of Russian Federation within framework of Government contract with Federal Research Center “Crystallography and Photonics” of Russian Academy of Sciences, particularly concerning with development methods for increasing the optical transparency of perfluoropolymers, and also within framework of RFBR grants No. 18-29-20102 and No. 16-29-05407, particularly concerning with synthesis of copolymers and creating polymer films, accordingly. The authors thank to Yu. E. Pogodin for help in synthesis of copolymers.
References
- Groh W. Overtone absorption in macromolecules for polymer optical fibers // Makromol. Chem. 1988, 189, 2861-2874.
- Eldada L., Shacklette L.W. Advances in polymer integrated optics // IEEE Journal of selected topics in quantum electronics. 2000, 6, 54-68.
- Sokolov V.I., Akhmanov A.S., Igoumnov S.M. at al. Development of the Element Base for High-Speed Integrated Optic Devices Based on Novel Polymer Materials // Vestnik RFFI. 2014, 3(83), 78-87. (in Russian)
- Sokolov V. I., Goriachuk I. O., Zavarzin I. V., at al. New copolymers of perfluoro-2-ethyl-2-methyl-1,3-dioxole and perfl uorovinyl ether with low non-monotonic refractive index // Russian Chemical Bulletin. 2019, 3, 559-564.
- Fischer D., Lappan U., Hopfe I., at al. FTi.r. spectroscopy on electron irradiated polytetrafluoroethylene // Polymer. 1998, 39, 573-582.
- Chatalova-Sazepin C., Binayeva M., Epifanov M., at al. Xenon Difluoride Mediated Fluorodecarboxylations for the Syntheses of Di- and Trifluoromethoxyarenes // Organic Letters. 2016, 18(18), 4570-4573.
ARTICLE INFO
Received 10 August 2019
Accepted 17 September 2019
Available online October 2019
Recommended for publication by Prof. S. Igumnov
Fluorine Notes, 2019, 126, 7-8
