Received: May 2019
DOI 10.17677/fn20714807.2019.04.05
Fluorine Notes, 2019, 125, 9-10
SYNTHESIS OF FLUORINE-CONTAINING LIGANDS OF BENZOFURAN TYPE AND PROPERTIES OF THEIR COMPLEXES WITH EUROPIUM (III) IN AQUEOUS SOLUTIONS
D. E. Pugachev 1,2, G. V. Zatonsky 2, N. V. Vasiliev1,2*.
1 Moscow State Regional University, 10-a Radio Str., Moscow, 105005, Russia
e-mail: nikolai-vasilev@mail.ru
2 FGUP State Scientific Research Institute of Biological Instrumentation, est. 1, 75, Volokolamsk
Highway, Moscow, 125424, Russia
e-mail: pugachovdmitry@gmail.com
Abstract: New types of fluorine-containing enaminones and triketones of benzofuran type have been synthesized in order to study their complexes with europium ion as potential luminescent reagents for the immunofluorescence analysis. The resulting compounds form complexes with europium (III) ion in aqueous solutions, but hydrolyze over time forming complexes of the corresponding β-diketones, which is traced by changing the spectral characteristics over time
Keywords: benzofuranyl trifluoromethyl containing complexones, Claisen reaction, enaminone, diketone, triketone, europium chelates, luminescence-spectral properties, immunofluorescence analysis
It is known that fluorine-containing complexones of aromatic and heteroaromatic types are widly used for lanthanide immunofluorescence analysis (LIFA). Fluorinated substituents provide increased stability of the resulting complexes and increase hydrophobic displacement of water from the inner sphere, which reduces the quenching of luminescence in aqueous solutions. It is also known that the benzofurane fragment provides optimal spectral characteristics, including good quantum sensitization in the long-wavelength region (≥350 nm). Thus, 2-benzofuranyltrifluoroacetone 2, obtained for the first time in [1], and its homologues form sufficiently stable complexes with europium in the presence of trioctyl phosphinoxide (TOPO), which exhibit significant fluorogenic properties [2, 3]. The purpose of the study was to investigate methods for producing fluorine-containing enamine-ketones and triketones of the benzofuran type and to research them as Eu3 + sensitizing complexones.
Fluorine-containing enaminones are tautomers of the corresponding imines and are rather well studied due to a series of works generalized in reviews [4-6]. These compounds are electronic analogues of enol forms of β-dicketons and exhibit good properties of complexones with respect to a number of metals [7-9].
2-Benzofuranyltrifluoroacetone 2, prepared by a modified procedure using lithium hydride as the main reagent, was used as the original reagent for the preparation of enaminones (Scheme 1).

Scheme 1. Preparation of benzofuranyltrifluoroacetone 2.
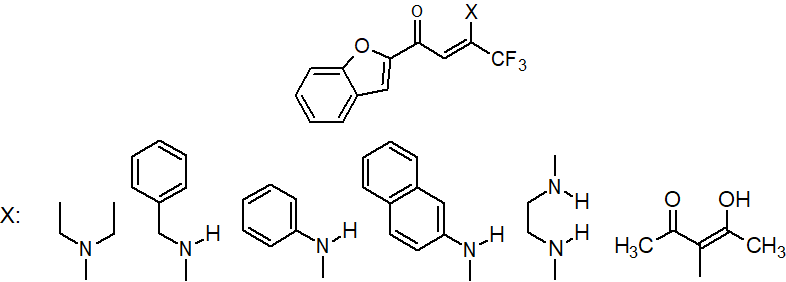
Direct reaction of amines with fluorinated β-diketones does not always make it possible to prepare imines or enaminones isomeric to them, and, as a rule, is carried out nonregioselectively [10]. Our attempts to synthesize enaminones 5 by the reaction of diketone 2 with primary amines also did not result in the isolation of individual compounds. Initially formed salts of amines 3 were subjected to secondary transformations when heated which resulted in the formation of resin-like products (Scheme 2).
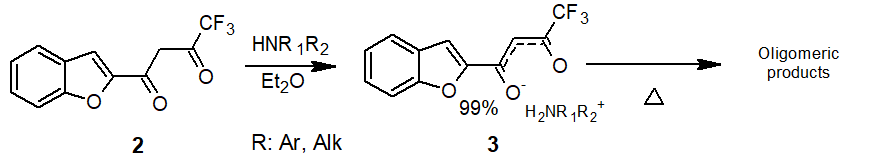
Scheme 2. Reaction of amines with β-diketone 2.
In this regard, a two-stage scheme, based on the intermediate use of chlorenone 4, was selected for the preparation of enaminone (Scheme 3). Chlorenone 4 was prepared in a satisfactory yield (83%) in the first stage by reaction diketone 2 with thionyl chloride in the presence of DMF catalytic amount. Benzofuranylchlorenone 4 is formed as low-melting crystals, which are easily purified by distillation in vacuum. Harenna 4 with amines reaction is carried out in dry diethyl ether at room temperature. In the case of aliphatic amines, their double excess was used as the acceptor of hydrogen chloride; for aromatic amines, triethylamine was used (Scheme 3). Yields of the resulting 5a-d compounds are quite high (≥78 %).
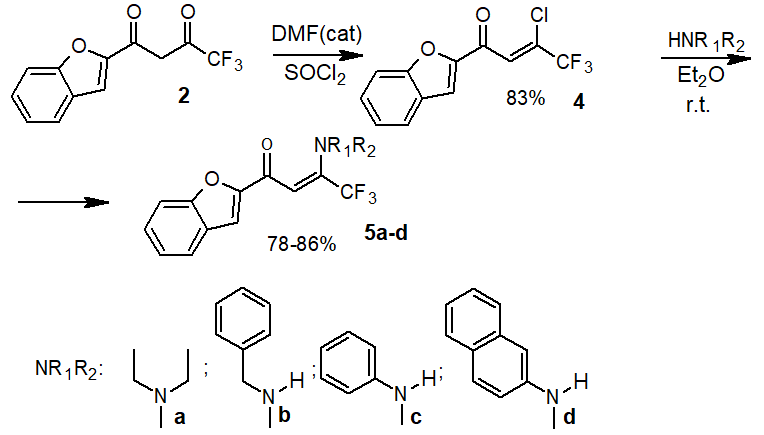
Scheme 3. Preparation of enaminones 5a-e.
To increase the chelating ability of the studied enaminones in the triethylamine environment, bis-substituted
enaminone 5e was prepared on the basis of ethylenediamine (Scheme 4).
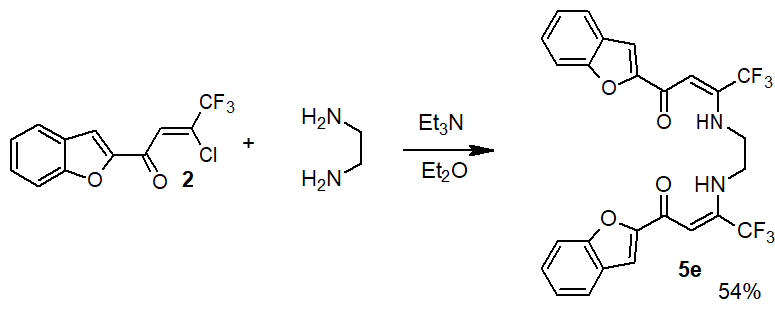
Scheme 4. Preparation of bis-enaminone 5e.
Enamine 5 is characterized by the presence of Z/E – isomerism fixed in the NMR spectrum, which was noted earlier for fluorine-containing enaminones [11–13]. 5a and 5e compounds are characterized by Z or E forms only, respectively.
Substitution of halogen in chlorasone 2 is also possible by acetylacetone generated carbanions (Scheme 5). The reaction occurs in the presence of triethylamine, while triketone 6 is formed as a result of the replacement of chlorine, which, judging by the NMR 1H spectrum, is in the enolic form. The reaction of chlorenone 2 with hexafluoroacetylacetone under the similar conditions could not be carried out due to the reduced nucleophilicity of the corresponding carbanion.
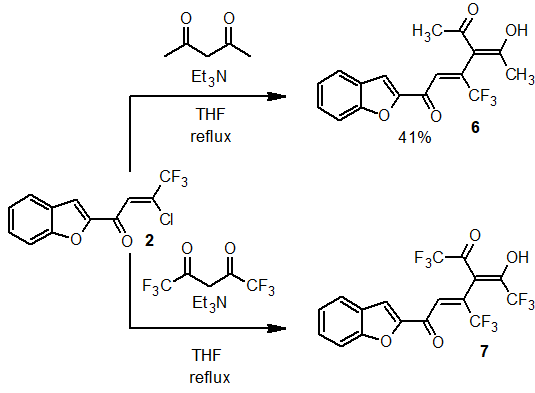
Scheme 5. Preparation of substituted acetylacetones 6, 7.
The absorption wavelengths of 5a-e enaminones for aqueous solutions in the UV and visible ranges are presented in Table 1. In comparison with the original diketone 2, enaminones are characterized by the presence of absorption bands in the longer wavelength region. Extinction of some compounds (5a, e) is comparable to or exceeds the extinction of diketone 2. With introduction of europium to the 3:1 ratio (except for compound 5e, for which, based on the ligand denticity, the 2:1 ratio was used) a bathochromic shift of absorption maxima or a change in optical density is observed for most of the compounds. The use of effective soligand trioctylphosphine oxide [14] also increases the degree of bathochromic displacement of the absorption bands by ~ 2-3 nm.
Table 1. Spectral properties of ligands 2, 5a-e, 6 and their Eu3+ complexes.
|
Ligand |
λ max(ε) nm(×104M-1×cm-1) |
+Eu3+ λmax(ε) nm(×104M-1×cm-1) |
|
2 |
352(2.67) |
356(2.61) |
|
5a |
338(2.70) |
338(2.36) |
|
5b |
357(2.08) 390(1.56) |
310(1.29) 370(0.96) 398(1.04) |
|
5c |
380(1.76) |
379(1.28) |
|
5d |
390(1.67) |
417(1.34) |
|
5e |
361(2.44) 399(2.89) |
363(2.02) 402(2.40) |
|
6 |
300(1.15) baseline 330(0.95) |
320 (0.91) |
The data presented in Table 1 indicate formation of complexes, which is confirmed by the additional experiments on the competitive displacement of ligands by the known 2-naphthoyltriftoracetone complexone. Luminescent properties of the complexes of compounds with europium ion in the ratio of 3:1 and 2:1 (for 5e) were determined in a water-micellar (0.1% Triton X-100) 0.05 M Tris buffer, both in the presence of TOPO and without it.
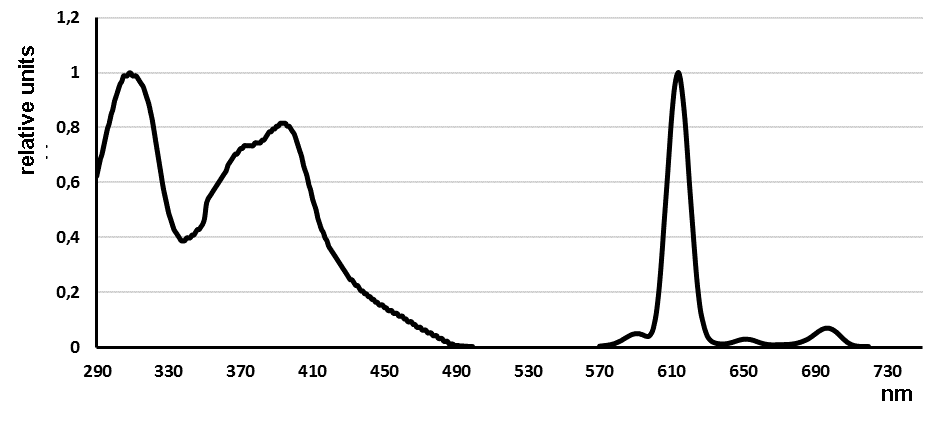
Figure 1. Absorption and luminescence spectra of complex 5b, (relative to the maximum value (for optical density of 0.129 (310 nm), for luminescence of 12,900 relative units (614 nm). С(Eu3+) = 3.3×10-6 М)).
In the emission spectra of europium complexes with fluorine-containing ligands 5 and 6 a typical pattern is observed – the main maximum at 614 nm (100 %) and minor maxima at wavelengths of 592 (5.0 %), 653 nm (3.0 %), 699 nm, (7.0 %) and 749 nm (<1.0 %) (Figure 1). In general, the luminescence of ligands complexes 5 and 6 in aqueous media is quenched in comparison with 2. At that, luminescence of the complexes solutions does not decrease but increases with time (Fig. 2), and at the same time, a hypsochromic shift of the luminescence excitation maximum occurs (Fig. 3).
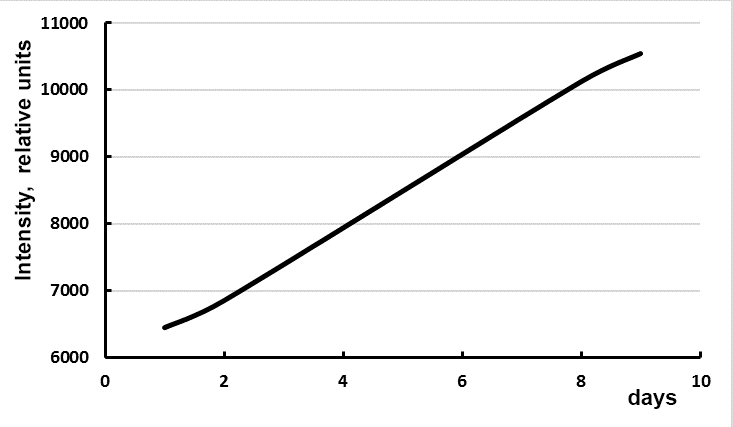
Figure 2. Change in the luminescence intensity of complex 5b with time (registration of Eu3+(3.3×10-6 М emission at 614 ±2 nm, excitation at 354 nm).
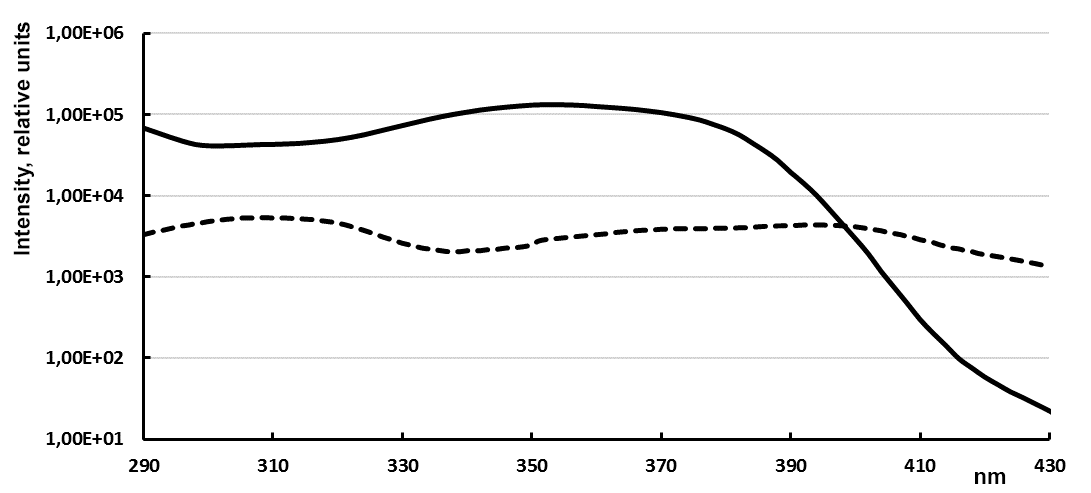
Figure 3. Changes in excitation spectrum of complex 5b in 9 days (dotted line – excitation spectrum on the 1st day, solid line – in 9 days; registration on Eu3+(3.3×10-6 М) emission at 614±2 nm, y-axis – in lg scale)
Thus, the obtained spectral data suggest that europium complexes with enaminones 5 undergo hydrolytic reactions in aqueous solutions and turn into complexes of the original diketone 2 (Scheme 6).
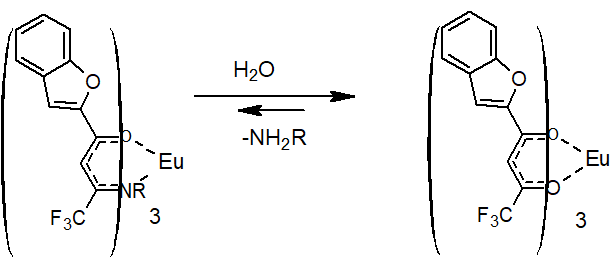
Scheme 6. Hydrolysis of enaminone 5 complex in accordance with spectral characteristics.
Synthesized in the course of the study fluorine-containing ligands of benzofuran 5, 6 type, as expected, form complexes with europium (III) ions. The complexes have significant extinction and moderate luminescence. Judging by the spectral data, it has been found that enaminones 5 complexes are susceptible to hydrolysis and turn into the complexes of fluorinated β-diketones. Thus, the peculiarity of the properties of these compounds determines the possibility of their further research in techniques related to biochipping in anhydrous environment.
Experimental procedure
NMR spectra of 1H, 19F solutions of substances in DMSO-d6 and CDCl3 were recorded on JNM-ECX400 spectrometer (with operating frequencies of 400 and 376 MHz). Chemical shifts are presented in ppm relative to the external standards: ТМS (trimethylsilyl) (1H); CFCl3 (19F). Elemental analysis was performed on Perkin Elmer CHN RE 2400 SII device. Melting points were determined in glass capillaries on Gallenkamp MPD350.BM3.5 apparatus. Absorption spectra were recorded on Shimadzu UV-1650PC in 1 cm quartz cuvette in the range of 290-500 nm. Luminescence of complexes with europium ion 2, 5a-e and 6 was recorded onVarioscan Flash microplate reader manufactured by Thermofisher Scientific with a time delay of 100 µs (td), during which the main luminescence of interfering substances was "cut off", and the integration time of 1000 µs (tg).
Commercial reagents and solvents prepared in accordance with known recommendations were used in the work [15]. 2-benzofuranylmethyl ketone (99 %), Sigma Aldrich was used.
1-(Benzofuran-2-yl)-4,4,4-trifluorobutane-1,3-dione (2)
(modified procedure)
The mixture of 2-benzofuranilmethyl ketone 1 (7.00 g, 43.80 mmol), LiH (1.05 g, 131.00 mmol) and CF3COOMe (10.10 g, 78.80 mmol) in dry THF (140 ml) is heated slowly to 60°С, and the heating is stopped after the start of exothermia. At the end of the exothermic reaction, the mixture is additionally refluxed for 1 hour, cooled to room temperature, the volatile components are evaporated under the reduced pressure (10 mm Hg). The reaction mixture is acidified with a cooled 3% hydrochloric acid solution (~0°C) to pH 2 and extracted with chloroform (3×90 ml). The organic layer is washed with water (2×50 ml), dried with Na2SO4, the solvent is evaporated under vacuum. The compound is used further without additional purification. Yield 10.81 g (96%), MP (melting point) = 72-74°C. According to the literature sources, MP = 74°C [3].
1-(Benzofuran-2-yl)-3-chloro-4,4,4-trifluorobut-2-ene-1-one (4)
DMF(100 µl, 1.96 mmol) is added to solution of diketone 2 (10.00 g, 39.10 mmol) in 100 ml of SOCl2 and refluxed for 3.5 h. SOCl2 is then distilled off in a vacuum of a water injection pump. The residue is dissolved in CHCl3 (150 ml) and washed with H2O (60 ml). The organic layer Na2SO4 is dried, the solvent is distilled in vacuum. The substance is distilled while collecting the fraction – 115-119°С (1 mm Hg). Yield 83%, MP =80-82°С. 1H NMR (400 MHz, CDCl3): δ 7.35 (ddd, 1H, arom., J = 8.0 Hz, 2.4 Hz, 1.2 Hz), 7.54 (dd, 1H, arom., J = 8.0 Hz, 2.4 Hz, 1.2 Hz), 7.61 (ddd, 1H, arom., J = 8.0 Hz, 1.2 Hz), 7.65 (d, 1H, 1.0 Hz), 7.67 (D, 1H, arom., J = 0.8 Hz), 7.75 (dd, 1H, arom., J = 8.0 Hz, 1.2 Hz). 19F NMR (376 MHz, CDCl3) δ -76.5 (s, CF3). Calculated for C12H6ClF3O2: С 52.48; Н 2.20. Found С 52.57; Н 2.09.
General procedure for preparation of enaminone (5a, b)
4 (0.22 g, 0.80 mmol) in dry diethyl ether (10 ml) is add dropwise to solution of the corresponding amine (2.08 mmol) in dry diethyl ether (5 ml), stirred at room temperature, controlling the course of the reaction by TLC (chloroform/hexane 3:1) for approximately 5-8 hours. After completion of the reaction, H2O (20 ml) is added, the organic layer is separated, washed with H2O (2x10 ml), dried with Na2SO4 and volatile components are distilled in vacuum.
1-(Benzofuran-2-yl)-4,4,4-trifluoro-3-diethylaminobut-2-ene-1-one (5a)
Precipitated from chloroform with hexane. Yield 82%, MP = 92-94 °С. 1H NMR (400 MHz, DMSO-d6): δ 1.10 (br. s, 3H, CH3), 1.25 (br. s, 3H, CH3.), 3.15 (br. s, 1H, CH2.), 3.55 (br. s, 1H, CH2), 5.51 (s, 1H, CH=), 7.09 (s, 1H, arom.), 7.28 (ddd, 1H, arom., J = 8.0 Hz, 2.4 Hz, 1.2 Hz), 7.35 (ddd, 1H, arom. J = 8.0 Hz, 2.4 Hz, 1.2 Hz), 7.60 (dd, 1H, arom., J = 8.0 Hz, 1.2 Hz), 7.67 (dd, 1H, arom., J = 8.0 Hz, 1.2 Hz). 19F NMR (376 MHz, CDCl3) δ -76.82 (s, CF3). Calculated for C16H16F3NO2: С 61.73; Н 5.18; N 4.50. Found С 61.56; Н 5.27; N 4.62.
1-(Benzofuran-2-yl) -4,4,4-trifluoro-3-benzylaminobut-2-ene-1-one (5b)
Recrystallized from 95 % ethanol. Yield 86%, MP =65-69°С. 1H NMR (400 MHz, DMSO-d6): δ 4.64 (d, 2H, CH2, J = 1.0 Hz), 6.33 (s, 1H, CH=), 7.28-7.39 (m, 6H, arom.), 7.47 (ddd, 1H, benzofur., J = 8.0, 2.4 Hz, 1.2 Hz), 7.68 (d, 1H, benzofur., J = 8.0, 1.2 Hz), 7.76 (d, 1H, arom., J = 8.0 Hz), 7.79 (d, 1H, arom., J = 8.0 Hz), 10.72 (br. s, NH). 19F NMR (376 MHz, CDCl3) δ -76.2 (s, CF3). Calculated for C19H14F3NO2: С 66.09; Н 4.09; N 4.06. Found С 66.00; Н 3.99; N 4.11.
General Procedure for Preparation of Enaminone (5c-e)
3 (0.22 g, 0.80 mmol) in dry diethyl ether (10 ml) is added to solution of the corresponding amine (0.80 mmol) and triethylamine (0.18 g, 1.80 mmol) in dry diethyl ether (5 ml), stirred at room temperature and controlling the course of the reaction by TLC (chloroform/hexacarbonyl 3:1). After completion of the reaction, H2O (20 ml) is added, the organic layer is separated, dried with Na2SO4 and the solvent is distilled in vacuum.
1- (Benzofuran-2-yl) -4,4,4-trifluoro-3-phenylaminobut-2-ene-1-one (5c)
Recrystallized from 95% ethanol. Yield 78%, MP =112-114°C. 1H NMR (400 MHz, DMSO-d6): δ 6.19 (s, 1H, CH=.), 7.12 (s, 1H, benzofur.), 7.2-7.3 (m, 4H, arom.,), 7.31-7.39 (m, 3H, arom.), 7.49 (dd, 1H, benzofur., J = 8.0 Hz, 1.2 Hz), 7.65 (dd, 1H, benzofur., J = 8.0 Hz, 1.2 Hz), 12.00 (br. s, NH). 19F NMR (376 MHz, CDCl3) δ -76.50 (s, CF3). Calculated forC18H12F3NO2: С 65.26; Н 3.65; N 4.23. Found С 64.97; Н 3.55; N 4.14.
1- (Benzofuran-2-yl) -4,4,4-trifluoro--3- (2-naphthylamino) but-2-ene-1-one (5d)
Recrystallized from 95% ethanol, yield 82%, MP(decomp.) = 105-108 °С. 1H NMR (400 MHz, DMSO-d6): δ 6.21 (s, 1H, Z CH=), 6.50 (s, 1H, E CH=), 7.1-7.9 (m, 24H), 11.62 (br. s, 1H, E-NH), 12.12 (br. s, Z-NH). 19F NMR (376 MHz, CDCl3) δ -76.32 (d, E-CF3), -76.49 (d, Z-CF3). Calculated for C22H14F3NO2: С 69.29; Н 3.70; N 3.67. Found С 69.35; Н 3.83; N 3.55.
N,N'-bis(3-(benzofuran-2-yl)-1-trifloromethyl-3-oxoprop-1-ene-1-yl)-1,2-diaminoethane (5e)
Recrystallized from 95% ethanol, yield 54%, MP = 167-169°С. 1H NMR (400 MHz, CDCl3): δ 3.73 (m, 4H, CH2,), 6.44 (s, 2H, CH=), 7.31 (ddd, 2H, arom., J = 8.0 Hz, 2.4 Hz, 1.2 Hz), 7.46 (ddd, 2H, arom., J = 8.0 Hz, 2.4 Hz, 1.2 Hz), 7.48 (s, 2H, arom.), 7.58 (dd, 2H, arom., J = 8.0 Hz, 1.2 Hz), 7.60 (dd, 2H, arom., J = 8.0 Hz, 1.2 Hz), 10.72 (br. s, 2H, NH). 19F NMR (376 MHz, CDCl3) δ -66.24 (s, CF3). Calculated for C26H18F6N2O4: C 58.22; H 3.38; N 5.22. Found С 58.16; Н 3.45; N 5.31.
1-(Benzofuran-2-yl)-4-(1-hydroxyethylidene)-3-(trifluoromethyl) hex-2-ene-1.5-dione (6)
To a solution of acetylacetone (0.25 g, 2.50 mmol) in 10 ml of diethyl ether was added triethylamine (0.27 g, 2.67 mmol) in 4 ml of diethyl ether under cooling. After added chlorenone 4 (0.68 g, 2.50 mmol) in 6 ml of diethyl ether. Stirred at room temperature for 2 hours, then refluxed for 18 hours. Then the precipitated salt Et3N×HCl is filtered. The filtrate was evaporated under reduced pressure. Dissolved in boiling hexane, filtered and removed to crystallize at -18°С. Filtered, washed with ice-cold hexane. Yield 41%, MP(decomp.) = 130-133°С. 1H NMR (400 MHz, DMSO-d6): δ 2.05 (s, 6H, CH3), 7.35 (ddd, 1H, arom., J = 8.0 Hz, 2.4 Hz, 1.2 Hz), 7.55 (ddd, 1H, arom., J = 8.0 Hz, 2.4 Hz, 1.2 Hz) 7.74 (dd, 1H, arom., J = 8.0 Hz, 1.2 Hz). 19F NMR (376 MHz, CDCl3) δ -67.963 (s, CF3). Calculated for C17H13F3O4: С 60.36; Н 3.87. Found С 60.41; Н 3.83.
General Procedure for Preparing Solutions of Enaminone (5a-f) Complexes with Eu3+ and TOPO in a Water-Micellar Tris Buffer
The required amount of ligand (5a-e) is dissolved in 2 ml of DMF with the formation of solution with concentration of 5×10-3 M. 100 μl of aliquot is taken and diluted with 0.05 M Tris buffer (pH 7.2) to the concentration of a ligand of 10-4 M. Freshly prepared solution of 10-4 M Eu3+ (EuNO3) is added to 10-5 М НСI,10-3 М TOPO in ethanol and the required quantity of Tris-buffer to prepare the final solution of the ligand: Eu:TOPO = (1.00:0.33:1.00)x10-5 M, which is left for 2 hours with no lighting to form a complex. To prepare the micellar solution, 10% Triton X-100 solution in ethanol is added to the original Tris buffer solution up to a final SAS concentration of 0.1 %. 200 µl is withdrawn from the solutions of the complexes to study the luminescent spectral characteristics.
References
- B. Barkley, R. Levine, J. Amer. Chem. Soc., 1951, 73, 4625-4627.
- T. S. Kostryukova, N. P. Ivanovskaya, G. V. Zatonskii, N. S. Osin, N. V. Vasil’ev, Russ J Bioorg Chem., 2015, 41, 186-191.
- D. V. Romanov, A. I. Ljamin, N. P. Ivanovskaja, A. E. Zhedulov, N. S. Osin, N. V. Vasil’ev, Pat. RU 2373200.
- A-Z. A. Elassar, A. A. El-Khair, Tetrahedron, 2003, 59(43), 8463-8480.
- S. V. Druzhinin, E. S. Balenkova, V. G. Nenajdenko, Tetrahedron, 2007, 63, 7753-7808.
- V. G. Nenajdenko, E. S. Balenkova, Reviews and Accounts, 2011, 1, 246-328.
- M. Y. Khuhawar, A. G. Bhatti, J. Chromatography, 1991, 558, 187-195.
- E. F. Zhilina, P. A. Slepukhin, N. S. Boltacheva, M. G. Pervova, D. L. Chizhov, V. I. Filyakova, V. N. Charushin, Russ. J. Gen. Chem., 2012, 82(12), 1962-1969.
- S. D. Cosham, G. Kociok-Köhn, A. L. Johnson, J. A. Hamilton, M. S. Hill, K. C. Molloy, R. Castaing, Eur. J. Inorg. Chem., 2015, 26, 4362-4372.
- K. I. Pashkevich, V. I. Saloutin and I. Y. Postovskii, Russ. Chem. Rev., 1981, 50 180-195.
- A. L. Krasovsky, V. G. Nenajdenko, E. S. Balenkova, Russ. Chem. Bull., 2001, 50(8), 1395-1400.
- I. H. Jeong, S. L. Jeon, Y. K. Min, B. T. Kim, Tetrahedron Lett., 2002, 43, 7171-7174.
- N. M. D. Brown, D. C. Nonhebe, Tetrahedron, 1968, 24, 5655-5664.
- D. E. Pugachov, T. S. Kostryukova, G. V. Zatonsky, S. Z. Vatsadze, N. V. Vasil'ev, Chem. Heterocycl. Compd., 2018, 54, 528-534.
- The Chemist’s companion. Gordon A. J.; Ford R. A.; New York-London-Sydney-Toronto. A Wiley-interscience publication, Jonh Wiley and Sons, 1972, 560 p.
ARTICLE INFO
Received 23 May 2019
Accepted 25 July 2019
Available online August 2019
Recommended for publication by Prof. S.M. Igumnov
Fluorine Notes, 2019, 125, 9-10
