Received: March 2019
DOI 10.17677/fn20714807.2019.03.04
Fluorine Notes, 2019, 124, 7-8
NMR Analysis in the technology of perfluoro(vinyloxy)butanoic acid ester production
Pospelova N.B. 1, Mokrushin I.G.2, Galeev A.R. 2, Tiunov A.V. 3
1Federal State Unitary Enterprise "Russian Scientific Center" Applied Chemistry"
2Perm State National Research University, Perm, Russia
3"Promchimperm", Perm, Russia.
Abstract: Spectrum characteristics of fluorine compounds at all stages of perfluoro(vinyloxy)butanoic acid methyl ester synthesis, including fluorosulfatizing mixture obtainment and hexafluoropropylene oxidation stage were described and clarified. Mixtures in various ratios were analyzed. Approaches for reactive compounds recording and methodology for quantitative evaluation of mixture content on each manufacturing stage were described.
Keywords: nuclear magnetic resonance, NMR fluorine-19, perfluorinated compounds, hexafluoropropylene oxide, fluorosulfatizing mixture, membranes’ monomers.
Introduction
Electrochemical ion-exchange membranes are applied almost across the board – from a technology for caustic soda production up to lithium batteries in mobile phones. Fluorine compounds, for example, perfluoro-4-(vinyloxy) butanoic acid methyl ester, which is produced on the base of the Federal State Unitary Enterprise "Russian Scientific Center" Applied Chemistry", can be used as a monomer for such membranes. The steps for a monomer preparing operate with compounds having high corrosive activity, which excluded the usage of traditional chromatographic analysis procedure. The simplest experiment NMR – recording of a panoramic spectrum with resonant lines integration, allowed to establish structure of nearly all obtained compounds, either of the main reactions, or of side reactions. The qualitative and the quantitative content of the products were controlled by 19F NMR.
Mixtures were analyzed on Bruker WP80SY 80 MHz spectrometer. Chemical shifts in 19F spectra were taken in ppm from CFCl3 using hexafluorobenzene (-162.90 ppm) as a standard. Signals of solvents are taken as standards in H1NMR spectra (δ, ppm, from TMS): 2.05 - acetone-D6; 2.50 - DMSO-D6, 7.26 - CDCl3.
While conducting the quantitative NMR analysis of sulphonyl fluorides in a mixture with starting compounds
and admixtures, which are formed either during the electrochemical fluorination, or electrolysis,
it is necessary to integrate each particular signal or signal group [1, 2]. Next, to sum the obtained
data Σ(I)=I1+I2+…+In. Integrated area
of the resonance line Ia is found separately, a is the quantity of fluorine atoms that gave it, n is the total quantity
of atoms, which gave a signal, in the determined molecule. Molar content is found according to the
formula:
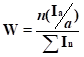
Chemical shifts, which are used as analytical, on the further schemes are marked with «*». «Analytical» groups signals allow to assume the presence of a compound in a mixture and «compile a molecule» by integral intensities of other parts. Standard statistic processing of results was held: abnormality check, belonging to normal distribution; allowable discrepancy determination between parallel definitions, random component error boarders, maximum systematic inaccuracy.
Literature review
Perfluorinated vinyl esters have been used as co-monomers for formation of curing sites in fluorinated polymers and as monomers for producing fluorine-containing polymers with special functions, such as fluoroelastomer and cation-exchange groups, in a membrane with selective permeability in the production of chlorine and caustic soda. Methods of synthesizing perfluorinated vinyl esters having an ester group are disclozed in patents [3,4]. The general methods include the following laborious reactions: (1) esterification of perfluorodiacyl fluoride and hexafluoropropylene oxide to give perfluoro(2-methyl-3-oxaalkane) diacylfluorides on cesium fluoride (2) solvolysis and synthesis of dimetal salt and (3) thermal synthesis decomposition and esterification to produce the desired product with a relatively low yield .
An easy and efficient synthesis for perfluorinated vinyl esters having ester group is described in the article by Yamabe with colleagues by pyrolysis of asymmetrical diacyl fluoride having FC(O)–CF(CF3)–O– group on one end and FC(O)–CF2– group – on the other end, at the temperature of about 290–370 °C in gaseous phase without loss of –COF group [5].
Results and Discussion
The scheme for the preparation of the monomer and the spectral characteristics of the compounds, obtained
during the synthesis steps, are shown in the Picture 1. Compound 5 is prepared by
known method using potassium fluoride followed by pyrolysis [ 6 ]. Depending on the composition of
the mixture, the chemical shifts of the initial halocarbon-318 lie in the region of -68 ppm (-CF
2CF 2Cl ) and -119 ppm (-CF2CF2Cl); formed carbonyl difluoride
COF2 -19 -22 ppm. Analysis time 25 -30 min, the total error of the determination of the
major component does not exceed 3%. For the spectra of the AB type, the chemical shift of the center
of the line is given.
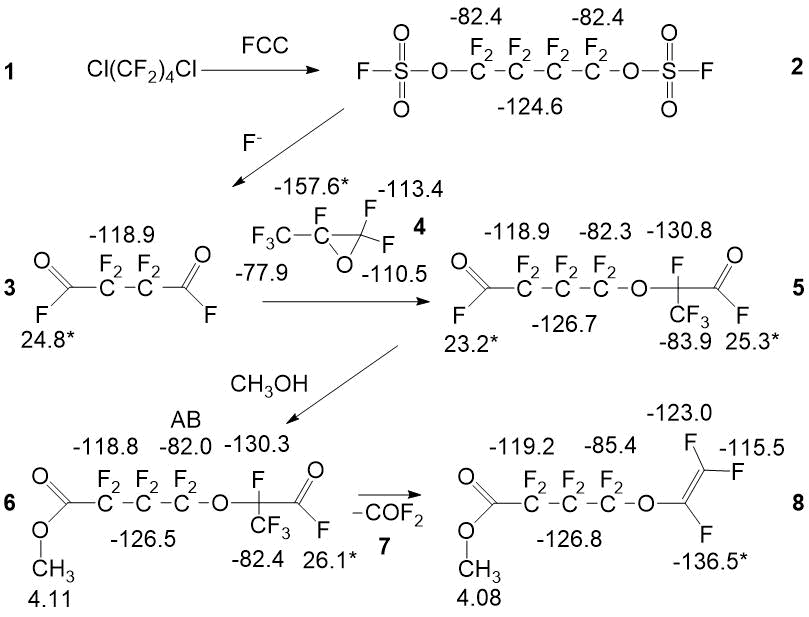
Picture 1. Scheme for monomer obtainment and spectral characteristics of the formed compounds.
The first step for the production of a monomer is the preparation of a fluorosulfating mixture (FCC) based on sulfonic acid fluoroanhydrides [7, 8]. NMR method for fluorosulfate mixture analysis was chosen because the given mixture has high reactive and corrosive activity, so the possibility of chromatographic analysis is nearly excluded. Analysis by NMR includes spectra recording of 0,5 ml of a mixture in a 5 mm fluoroplastic ampoule with an inner capillary with deuteroacetone or deuterodimethylsulfoxide with additive component - hexafluorobenzene as a standard. A single-use of standard glass ampoules is possible. The fluorosulfate mixture analysis time is 30-40 min., ultimate total error of basic components definition does not exceed 2 % re. Spectrum characteristics of fluorosulfate mixture components, such as chlorine fluorosulfat, chlorine fluoropyrosulphate, di(fluorosulfuryl)oxide, di(fluorosulfuryl)peroxide, fluorosulfonic acid, are shown in the Picture 2.
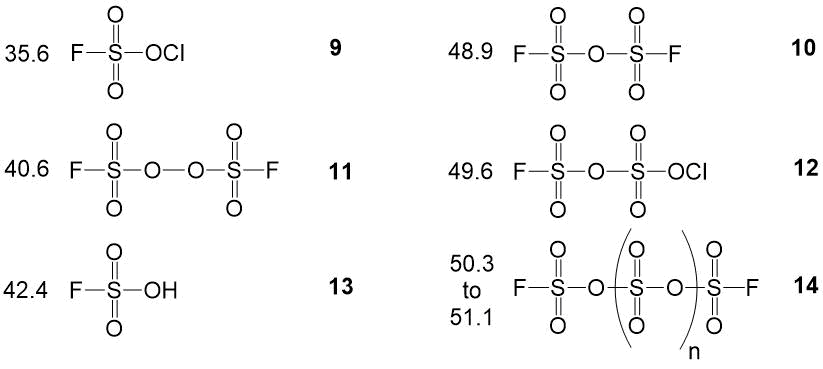
Picture 2. Spectrum characteristics of fluorosulfate liquid.
NMR spectra of hexafluoropropylene oxide and the found admixtures are worth to describe separately [9].
Fluorine CF-CF3 chemical shift is used as analytical. Fluorine NMR signals position in
hehafluoropropylene oxide strongly depends on a mixture content and may vary 157,6±3 ppm. Used methodology
of NMR analysis for technological hexafluoropropylene oxide includes bubbling of gaseous products
of hexafluoropropylene oxidation through 1 ml of deuterochloroform with addition of standard hexafluorobenzene
in a glass ampoule (5 mm) during 2-2,5 min. with a speed 120-180 ml/min. Accurate quantification
of the 2,2,3-trifluoro-3-(trifluoromethyl)oxirane obtained becomes complicated not only because of
physical and chemical characteristics of investigated products, but also due to the missing data
for solubility of hexafluoropropylene oxide and admixtures in deuterated solvents. Spectrum characteristics
of the found products are shown in the picture 3. Chlorinated derivative 11 was
found as an admixture, and it was probably formed during sample preparation for analysis.
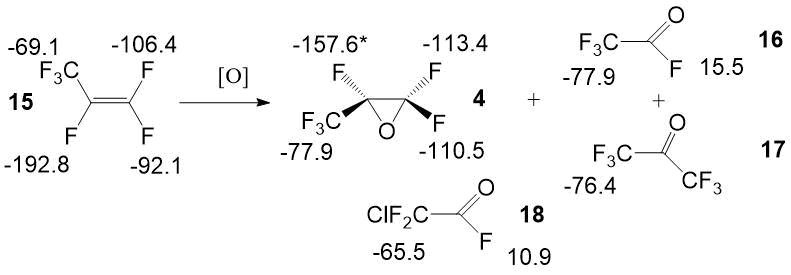
Picture 3. Spectrum characteristics of hexafluoropropylene oxide and found admixtures.
In the Picture 4 there are shown spectrum characteristics of the range of byproducts,
which were found during the elaboration of the technology.
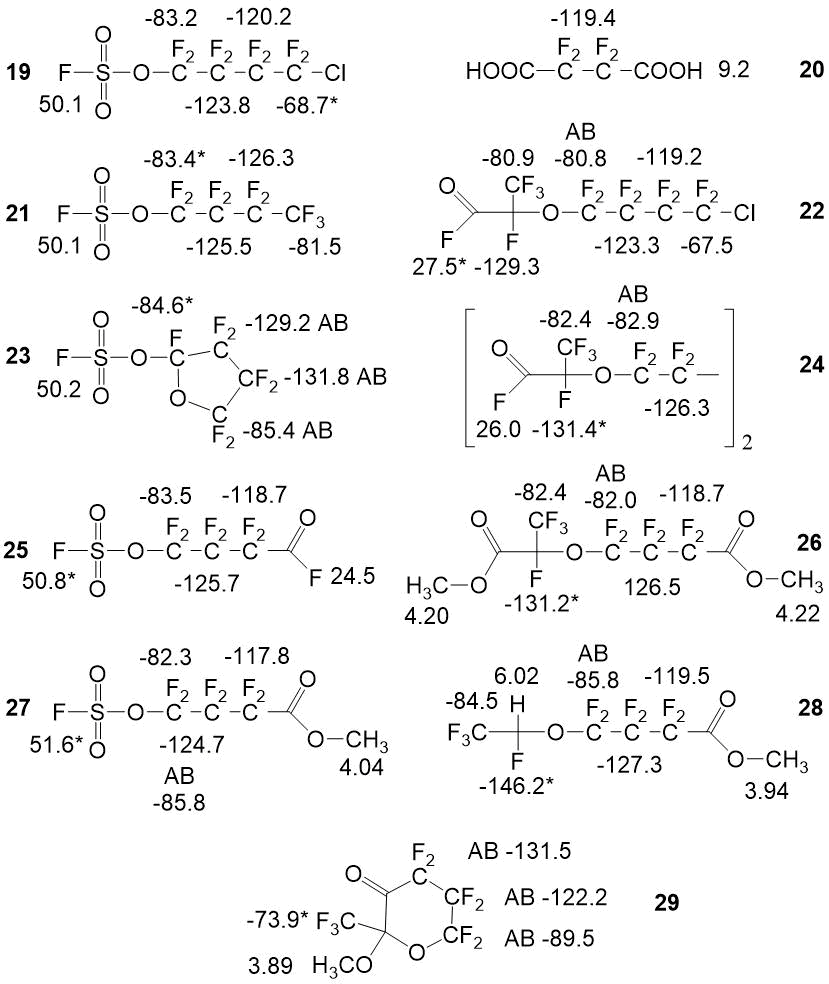
Picture 4. Spectrum characteristics of admixtures, which were found during the target monomer synthesis stages.
Conclusion
The NMR method can be used not only to establish the structure of the resulting compounds, but also as as a reference method at deviation of the process of synthesis of the methyl ester of perfluorovinyloxybutanoic acid from the preset process parameters. The publication describes methods of quantitative determination of the composition of highly reactive fluorine-containing compounds.
We have described the spectral characteristics and the procedure for the quantitative analysis of the following compounds: 1,4-dichloroperfluorobutane 1, 1,4-bis-fluorosulfonyloxyperfluorobutane - 2, succinic acid difluoride 3, hexafluoropropylene oxide 4, 4-(3-oxoperfluoropropane-2-yl)oxy-perfluorobutanoic acid fluoride 5 and it’s methyl ester 6, carbonyldifluoride 7, perfluorio-4-(vinyloxy)butanoic acid methyl ester 8, chlorine fluorosulfate 9, oxydisulfurylfluoride 10, bis-sulfurylfluoride peroxide 11, chlorine fluoropyrosulphate 12, fluorosulphonic acid 13, polysulfuric acids difluorides 14, hexafluoropropylene 15, trifluoroacetyl fluoride 16, hexafluoroacetone 17, difluorochloroacetyl fluoride 18, 4-chloro-1-fluorosulfonylperfluorobutane 19, perfluoroamber acid 20, 1-fluorosulfonyloxyperfluorobutane 21, 4-chloro-1-fluorosulfonyloxy-perfluorobutane 22, 2-fluorosulfonylperfluorofuran 23, 1,4-bis((3-oxopropane-2-yl)oxy)perfluorobutane 24, 4-fluorosulfonylperfluorobutanoic acid fluoride 25, 4-(3-methoxy-3-oxoperfluoropropane-2-yl)oxy-perfluorobutanoic acid methyl ester 26, 4-fluorosulfonylperfluorobutanoic acid methyl ester 27, 4-(1-hydroperfluoropropane -2-yl)oxy-perfluorobutanoic acid methyl ester 28, 2-methoxy-2-(trifluoromethyl)dihydro-2H-perfluoropyran-3(4H)-one 29.
References
- Dolbier, W.R. Guide to Fluorine NMR for Organic Chemists. John Wiley & Sons, 2006. 235 p.
- Pospelova N.B., Mokrushin I.G. Osobennosti YaMR analiza perftorirovannykh soedineniy [Experience of NMR analysis of perfluorinated compounds] // Vestnik Permskogo universiteta. Seriya «Khimiya» – Bulletin of Perm University. CHEMISTRY. 2016, 3(23). 85–91. (In Russ.). DOI: 10.17072/2223-1838-2016-3-85-91.
- Patent US2713593A, 1955.
- Patent US3682872A, 1972.
- Yamabe M. et al. Synthesis of perfluorinated vinyl ethers having ester group. Journal of Fluorine Chemistry. 1999, 94, 1, 65-68.
- Molchanov A.P., Kostikov R.R. Product subclass 2: 1-halo-1-(organooxy)alk-1-enes. Sci. Synth. 2006. 24, 129–166.
- Patent SU1787932A1, 1993.
- Patent US3536733, 1970.
- Patent RU2169730C1, 2001.
Recommended for publication by Prof. S.M. Igumnov
Fluorine Notes, 2019, 124, 7-8
