Received: May 2019
DOI 10.17677/fn20714807.2019.03.03
Fluorine Notes, 2019, 124, 5-6
Preparation of 2-Bromoethyl Tetrafluorochlorosulfate for the Synthesis of Bromodifluoroacetic Acid Derivatives
V.E. Boyko, V.L. Don, S.M. Igoumnov
A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Vavilov St. 28, GSP-1, Moscow, Russian Federation
e-mail: boykii@mail.ru
Abstract: Two-step process for preparing bromodifluoroacetate from tetrafluoroethylene suggested. Tetrafluoroethylene was reacted with bromine chloride in chlorosulfonic acid to give 2-bromotetrafluoroethyl chlorosulfate, which is converted into ethyl bromodifluoroacetate in ethanol under the action of a base in good yield.
Keywords: ethyl bromodifluoroacetate, 2-bromotetrafluoroethyl chlorosulfate
Ethyl bromodifluoroacetate is an important starting material for the synthesis of many pharmaceuticals as well as plant protection agents [1-3], therefore there is a need for new approaches to its production. We suggested the preparation of ethyl bromodifluoroacetate from 2-bromotetrafluoroethyl chlorosulfate.
2-Bromotetrafluoroethyl fluorosulfate was previously prepared by reacting 1,2-dibromo-tetrafluoroethane with bromofluorosulfate [4], or by reacting tetrafluoroethylene with bromosuccinimide and fluorosulfonic acid [5]. Bromofluorosulfate and fluorosulfonic acid are highly aggressive and hardly accesible reagents. We have found that tetrafluoroethylene reacts with bromine chloride in chlorosulfonic acid similar to that it reacts with iodine chloride in chlorosulfonic acid [6] to form 2-bromotetrafluoroacetyl chloride, not described previously in the literature. 2-Bromotetrafluoroethyl chlorsulfate thus obtained can be readily converted to various bromodifluoroacetic acid derivatives. The preparation of 2-bromotetrafluoroethyl chlorosulfate was carried out in an open system, without the use of pressure reactors. It should be noted that the preparation of 2-iodotetrafluoroethyl chlorosulfate also has a good yield in an open system, and there is no need to apply pressure reactors as described in [6] and [7].
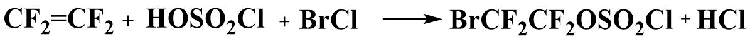
Bromine chloride was obtained by passing gaseous chlorine into liquid bromine under cooling, at the temperature below 0°C (bromine chloride is stable at temperatures below 5°C). Chlorosulfonic acid was then added to the reaction mixture and then tetrafluoroethylene was bubbled into the reaction mixture while maintaining the temperature within the range of -10°C to 0°C during the entire process. The resulting product was distilled off from the reaction mixture under vacuum and then distilled at atmospheric pressure. Distillation afforded 2-bromotetrafluoroethyl chlorosulfate in more than 90% yield as a clear liquid with b.p. 112°C.
Various derivatives of bromodifluoroacetic acid (esters, acyl fluoride, amide) can be prepared from 2-bromotetrafluoroethyl chlorosulfate, similar to the preparation of the iododifluoroacetic acid derivatives described in [7].
By reacting of 2-bromotetrafluoroethychlorsulfate (or 2-bromotetrafluoroethyl fluorosulfate) with ethyl
alcohol in the presence of a base under cooling, ethyl bromodifluoroacetate was obtained in good
yield.
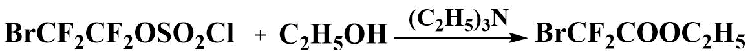
Most of the known processes for the production of ethyl bromodifluoroacetate are based on the reaction of bromodifluoroacetyl halides with ethyl alcohol, the acid halides themselves are prepared commonly by hydrolysis of various 1,1-difluorotetrahaloethanes-CF2BrCFClBr, CF2BrCBr2 or CF2BrCF2Br with oleum under catalysis of mercury compounds [8-10]. Chinese patent 2017 [11] describes the oxidation of 1,1-difluorotetrahaloethane with ozone followed by reaction of the oxide obtained with an alcohol to give ethyl bromodifluoroacetate. There is known a method in which various 1,1-difluorotetrahaloethanes are mixed with an alcohol and with radical initiator and further oxidized directly with gaseous oxygen diluted with some inert under heating, to yield ethyl bromodifluoroacetate [12].
The proposed two-step process for preparing bromodifluoroacetate from tetrafluoroethylene is convenient for use in laboratory conditions, since no special equipment requires, and can also be scaled for industrial production.
The preparation of ethyl bromodifluoroacetate is provided by the Russian federation patent no. 2602238.
Experimental Part
NMR 1H, 19F spectra were recorded on a “Bruker AVANCE-300” spectrometer at 300 and 282 MHz, respectively, exterior standard CDCl3. Chemical shifts for 1H spectra were determined relatively to proton signal of solvent (CDCl3) and given in ppm relative to TMS Chemical shifts of 19F spectra were determined in ppm relatively to CFCl3. Downfield shift are positive.
2-Bromotetrafluoroethyl chlorosulfate
Into a 2-liter four-necked round-bottom flask equipped with stirrer, thermometer, bubbler, condenser of deep cooling, filled with a mixture of acetone with dry ice, Tishenko bottle with H2SO4 at the outlet, bromine (230.4 g, 1.44 mol) was placed and cooled in an acetone-dry ice bath to a temperature of -10°C then chlorine is bubbled with vigorous stirring in this manner, so that the temperature of the reaction mixture does not rise above 0°C The end of the process is determined by the weight of the reaction mass (calculated amount of chlorine 102.2 g (1.44 mol) total reaction mixture weight 332.6 g). The reaction mass was then stirred for about 30 minutes to complete the reaction.
At 0 -10ºC under stirring and cooling chlorosulphonic acid (685 g, 5.9 mol) was added to the reaction mixture and then tetrafluoroethylene (350 g, 3.5 mol) was bubbled into the reaction mixture, the product was distilled off under vacuum to the boiling point 50°C at 50 mm Hg (chlorosulfonic acid remains in the cube), and rectified. 2-Bromotetrafluoroethyl chlorosulfate (940 g, 91% yield) was obtained with a purity of 97%. Boiling point 112°C.
NMR 19F (δ, ppm.): -70,8, (m, 2F, CF2Br), -87,6 (m, 2F, CF2OSO2Cl)
Ethyl bromodifluoroacetate
Absolute triethylamine (0.8 g) was added under stirring to absolute ethanol (15.18 g, 0,33 mol) and 2-bromotetrafluoroethylchlorosulfate (32 g, 0.11 mol) was added dropwise with cooling to 0 -5°c and stirring. A further 4.75 g of triethylamine was then added at the same temperature. (5. 55g (0.055 mol) of triethylamine was added total). After the addition of the reagents was complete, the water bath was removed and the reaction was allowed to warm to room temperature, whereby a gas evolution occurred. Then 70 ml of methylene chloride and water were added to the reaction mixture. The organic layer was separated, the methylene chloride was separated by distillation with a reflux condenser, then the product was distilled off under vacuum and redistilled. Ethyl bromodifluoroacetate (15 g, 69% yield) was obtained with a purity of 97%.
B.p. 111-113°С, NMR 1H (δ, ppm.): 1,5 (t., J=7,0 Hz, 3H, CH3), 4,5 (q., J=7,0 Hz, 2H, CH2), NMR 19F (δ, ppm.): -62,12 (s, CF2Br).
Acknowledgments
This work was performed with the financial support from Ministry of Science and Higher Education of the Russian Federation using the equipment of Center for molecular composition studies of INEOS RAS.
References
- Konas, D.W, Coward, J. K., e-EROS Encyclopedia of Reagents for Organic Synthesis, 2005, 1-5.
- Tarui A., Ikebata T., Sato K., Omote M., Ando A., Org.Biomol. Chem., 2014, v. 12(33), 6484-6489.
- Patent application WO2014004064.
- Fokin A.V., Studnev Yu.N., Rapkin A.I., Tatarinov A.S., Seryanov Yu.V., Bull. Acad. Sci. USSR (div. chem. sci.) (Rus.Chem.Bull.), 1985, V.34, (7), pp 1495–1498.
- German L.S., Savicheva G.I., Bull. Acad. Sci. USSR (div. chem. sci.) (Rus.Chem.Bull.), 1984, 33(2), 447.
- Patent US5763552 (1998).
- Hung Ming-H., Long Lu, Yang Zhen-Yu, JOC, 2004, 69, 198-201.
- Paleta O., Liska F., Posta A., Collect. Czech. Chem. Commun., 1970, 35(4), 1302-1306.
- Morel D., Dawans, Tetrahedron, 1977, 33(12), 1445-1447.
- Patent EP2867228 (2017).
- Patent CN104628559 (2017).
- Patent US5619023 (1997).
Recommended for publication by Prof. V.V. Kornilov
Fluorine Notes, 2019, 124, 5-6
