Received: March 2019
DOI 10.17677/fn20714807.2019.02.04
Fluorine Notes, 2019, 123, 7-8
Exploration of SNF-Approach toward Functionalized Nitronyl Nitroxides
Pavel Fedyushin,1 Larisa Gurskaya,1 Elena Panteleeva,1,2 Borislav Koshcheev,1 Alexander Maksimov,1 Tatyana V. Rybalova,1,2 Elena Zaytseva,1,2 Evgeny Tretyakov1,2 *
1N.N. Voroztsov Institute of Organic Chemistry, Pr. Lavrentjeva 9, Novosibirsk, 630090, Russia
2Novosibirsk State University, 2 Pirogova Str., Novosibirsk 630090, Russia
E-mail: tretyakov@nioch.nsc.ru
Abstract: A 4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole-3-oxide-1-oxyl lithium derivative was found to react with perfluoroaromatic compounds with formation of products of fluoro-atom substitution, namely fluorinated aryl(hetaryl)-substituted nitronyl nitroxides. The yields of the fluorinated nitroxides were acceptable in cases of reactions with functionalized perfluorobenzene and perfluoropyridine (25–60%), but was low in case of reaction with perfluorobiphenyl (~ 5%). Molecular and crystal structures of the obtained nitronyl nitroxides were solved by monocrystal X-ray diffraction analysis, and the nature of the radical was ascertained by EPR.
Keywords: Fluorinated Arenes and Hetarenes Nucleophilic Substitution; Nitronyl Nitroxides; X-ray Diffraction Analysis
Introduction
Open-shell organic compounds are promising components for next-generation electronic and spin devices because of their wide diversity of molecular design, structural flexibility and processability [1]. In the past few decades, substituted nitronyl nitroxides (R–NN) have been actively utilised as organic spin carriers that allowed to discover materials with unique spin-related properties such as purely organic magnetic switches [2], ferromagnets and ferrimagnets [1], magnets with negative magnetoresistance [3] and graphene-based magnetic polymers [4].
Nitronyl nitroxides are generally obtained via condensation of bis-hydroxylamines with various aldehydes (or polyaldehydes) and subsequent oxidation of the corresponding 1,4-dihydroxyimidazolidines [5]. Nevertheless, the reactions of aromatic aldehydes bearing highly electron-withdrawing substituents have sometimes been unsuccessful [5b, 6], probably due to the low stability of the condensation products. The direct introduction of the NN group into organic scaffolds using a nitronyl nitroxide non-substituted at position 2 (H–NN) as a synthetic unit is an alternative and promising way to access a diverse range of radical derivatives R–NN. Throughout the history of nitronyl nitroxide chemistry, numerous nucleophilic and cross-coupled reactions with metalated derivatives M–NN have been successfully carried out with various electrophiles to obtain functionalised R–NNs [7,8]. The using of the paramagnetic lithium derivative Li–NN was especially fruitful in context of preparation of functionalized nitronyl nitroxides (Scheme 1) [9].
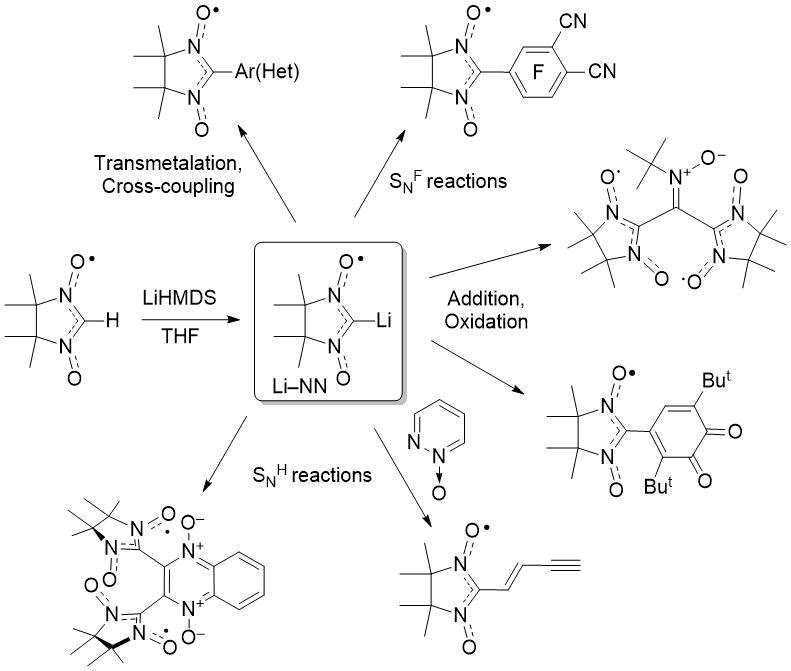
Scheme 1. Synthetic chemistry of lithiated derivative Li–NN.
Recently, we reported the possibility of fluorine substitution in perfluorobenzonitrile and perfluorophthalonitrile with Li–NN and thus prepared cyanotetrafluorophenyl- and dicyanotrifluorophenyl-substituted nitronyl nitroxides [10,11]. Here we explored the applicability of this reaction for the preparation of different polyfluorinated functionally-substituted arenes and hetarenes as well.
Results and Discussion
To carry out the reactions, Li–NN was generated by the action of lithium hexamethyldisilazane (LiHMDS)
on H–NN at –90 C in tetrahydrofuran (THF; Scheme 1) [12] and treated with the corresponding
perfluoroaromatic compound. After a relatively short period (4 h) the reaction gave a mixture of
products (TLC), from which the target product of fluorine atom substitution, respectively substituted
nitronyl nitroxides 1–4, were isolated using column chromatography with the subsequent
crystallization (Scheme 2).
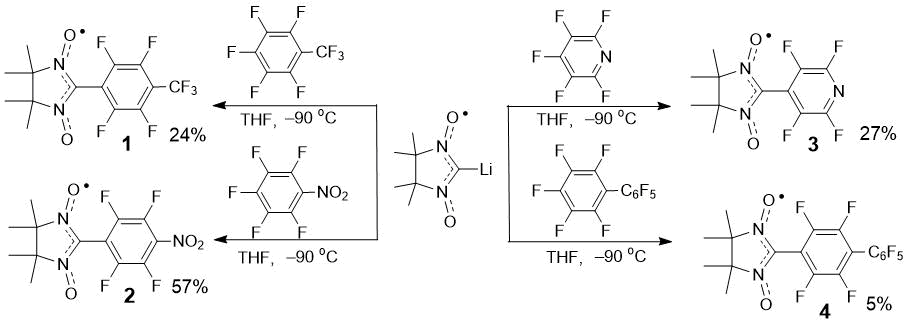
Scheme 2. Synthesis of substituted nitronyl nitroxides 1–4.
The structures of all nitronyl nitroxides were solved by single-crystal X-ray diffraction analysis (Figure 1). Selected geometrical parameters of compounds 1–4 are collected in Table 1. One can see that the bond lengths and bond angles in these nitronyl nitroxides are within the statistical means [13]; bond lengths CN and NO in the paramagnetic moiety are rather typical [7]. The dihedral angles between planes of the nitronyl nitroxide and aromatic moieties are within 57–70 range.
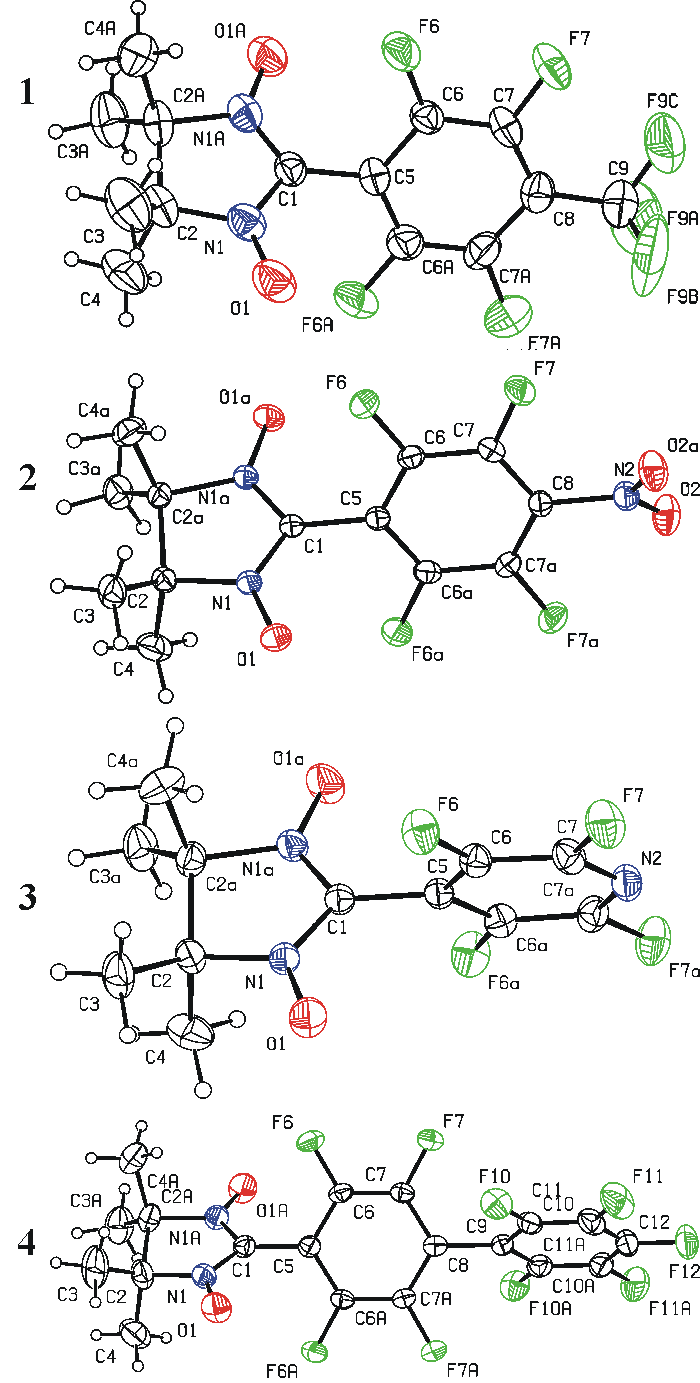
Figure 1. Molecular structures (ORTEP diagram with 30% ellipsoid probability) and atom numbering of nitronyl nitroxides 1–4.
Table 1. Selected geometrical parameters of compounds 1–4.
|
Bonds (Å) Angles (°) |
1 |
2 |
3 |
4 |
|
O1–N1 |
1.255(8) |
1.275(2) |
1.276(2) |
1.266(4) |
|
O1a–N1a |
1.272(8) |
1.275(2)* |
1.276(2)* |
1.277(4) |
|
N1–C1 |
1.362(8) |
1.336(2) |
1.337(2) |
1.339(5) |
|
N1a–C1 |
1.319(8) |
1.336(2) |
1.337(2) |
1.338(5) |
|
N1–C2 |
1.490(9) |
1.506(2) |
1.504(2) |
1.515(5) |
|
N1a–C2a |
1.528(9) |
1.506(2)* |
1.504(2)* |
1.501(5) |
|
C2–C2a |
1.586(9) |
1.573(3) |
1.546(2) |
1.571(6) |
|
C1–C5 |
1.456(9) |
1.470(3) |
1.469(3) |
1.465(5) |
|
O1-N1-C1 |
124.3(6) |
125.9(2) |
126.2(1) |
125.5(3) |
|
O1-N1-C2 |
122.4(5) |
122.1(1) |
123.1(1) |
123.2(3) |
|
C1-N1-C2 |
113.3(5) |
111.8(2) |
110.2(1) |
110.8(3) |
|
O1a-N1a-C1 |
125.1(5) |
125.9(2)* |
126.2(1)* |
125.9(3) |
|
O1a-N1a-C2a |
121.4(5) |
122.1(1)* |
123.1(1)* |
122.6(3) |
|
C1-N1a-C2a |
113.5(5) |
111.8(2)* |
110.2(1)* |
111.3(3) |
|
N1-C1-N1a |
109.7(5) |
110.9(2) |
110.7(2) |
110.6(3) |
|
α** |
57(1) |
70.3(2) |
69.9(2) |
60.4(6) |
* Molecules lay on 2-fold axis, so the bond lengths are same.
** Interplain angle for nitronyl nitroxide moiety and aromatic substituent.
The ESR spectra for diluted (10–4 M) and oxygen free chloroform solutions of 1–4 showed quintet-of-triplet patterns at g = 2.0060(1) (Figure 2a-e). In the case of 4 a high resolution ESR spectrum was recorded to give more complex splitting of the each line of its quintet. We attributed the quintet and triplet splitting to the two equivalent nitrogen nuclei and the two equivalent fluorine nuclei nearest to the nitronyl nitroxide fragment, respectively. The complex pattern observed for 4 was well reproduced taking into account 12 hfs constants on the protons of four methyl groups and two pairs of hfs constant on the distant fluorine atoms. The exact values of hfs constants used for the simulations are summarized in Table 2. It worth mentioned that despite the structural similarities of 1–4, the values of AN and AF are slightly different that suggests the differences in their electronic structures and the ESR spectra line shapes.
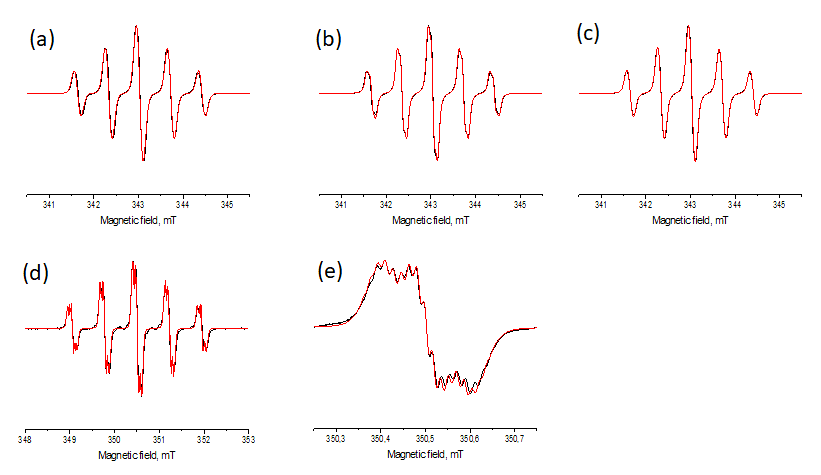
Figure 2. ESR spectra recorded for diluted and oxygen free chloroform solutions of 1 (a), 2 (b), 3 (c), 4 (the whole spectrum, d), 4 (the central component, e). Black lines – experimental spectra; red lines – computing simulations with parameters given in Table 2.
Table 2. Parameters used for the EPR spectra simulations.
|
Compound |
giso |
2AN,mT |
2AFortho, mT |
|
1 |
2.0060 |
0.71 |
0.07 |
|
2 |
2.0060 |
0.71 |
0.08 |
|
3 |
2.0060 |
0.71 |
0.07 |
|
4* |
2.0061 |
0.73 |
0.07 |
*For the simulation of high resolution ESR spectrum of 4 (Fig. 2e) the following values of hfs were used: 2AN = 0.73 mT; 2AF = 0.07 mT; 12AH = 0.015 mT; 2AF = 0.02; 2AF = 0.015 mT.
Conclusion
We explored the applicability of reaction of 4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole-3-oxide-1-oxyl lithium derivative with perfloroaromatic compounds for preparation of polyfluoroaryl(hetaryl)-substituted nitronyl nitroxides. It was revealed that in all cases the reaction occurs by regiospecific substitution of fluoro atom at the para-position relative to the functional group. All the synthesised radicals are stable and were completely characterised both in solution and in the solid state. The result opens a way to new nitronyl nitroxides with fluorinated electron-withdrawing substituents; such radicals in turn could be useful open-shell species for application in fields of molecular magnetism and material sciences.
Experimental Methods
1. Materials and Instrumentation
4,4,5,5-Tetramethyl-4,5-dihydro-1H-imidazol-3-oxide-1-oxyl (H–NN) [9b] was synthesised as reported earlier, THF was freshly distilled over benzophenone sodium ketyl. Other chemicals were of the highest purity commercially available and were used as received. Column chromatography was carried out on silica gel (0.063–0.200 mm). Infrared (IR) spectra were recorded by means of a Tensor 27 instrument for samples pelleted with KBr (0.25%). UV-vis spectra were registered on HP Agilent 8453 (10–5–10–4 M solutions in EtOH) spectrophotometers. Masses of molecular ions were determined by HRMS on a DFS Thermo scientific instrument (EI, 70 eV).
EPR spectra were acquired in a diluted and oxygen free chloroform solutions at 295 K at the concentrations of ~10–4 M by means of the commercial Bruker X Band (9 GHz) spectrometer Elexys E 540. For determining the isotropic g-factors (giso), we recorded X-band CW EPR spectra of mixture of the investigated radical with Finland trityl. Then the known giso of Finland trityl was used for the spectrum simulation, and the target giso value was excluded. The simulations of the solution EPR lines were carried out in the software package Easy Spin which is available at http://www.easypin.org.
2. Synthetic Methods and Characterization
General Procedure of Preparation of Nitronyl Nitroxides. A 1.0 M solution of LiHMDS (1.1 mL, 1.1 mmol) in THF was added at 90 C into a vigorously stirred solution of 4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazol-3-oxide-1-oxyl (157 mg, 1.0 mmol) in THF (20 mL) under argon. The reaction mixture was stirred at 90 C for 30 min. Then, the solution of perfluoroarene (1.1 mmol) in THF (5 mL) was added at –90 C in an argon atmosphere, stirring was continued, and the reaction was monitored by TLC (Silufol F254, EtOAc as eluent). After 4 h, TLC changes ceased, the cooling was stopped, and the reaction mixture was allowed to warm up to room temperature and brought into contact with the atmosphere. Flash chromatography (SiO2, column 3 × 4 cm, EtOAc as eluent) yielded a solid mixture after solvent removal under reduced pressure at room temperature. The resulting solid mixture was separated by column chromatography (SiO2, column 3 × 20 cm, CH2Cl2 as eluent), which produced a fraction of desired product. The fraction was concentrated under reduced pressure to a volume of ~5 mL, then n-heptane (5 mL) was added, and the mixture was incubated for ~60 h at 0–5 °C for slow crystallization of nitronyl nitroxide radical.
2-(4-Trifluoromethyl-2,3,5,6-tetrafluorophenyl)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazol-3-oxide-1-oxyle (1). Yield 90 mg (24%); violet crystals; IR (KBr) ṽ;max, cm−1: 409, 503, 536, 602, 677, 706, 719, 874, 970, 999, 1028, 1140, 1155, 1180, 1217, 1265, 1331, 1377, 1390, 1433, 1468, 1495, 1551, 1606, 1662, 2991, 3014, 3441; UV-vis (EtOH) max/nm (lg ): 553 (2.66), 373 (3.77), 294 (4.04), 203 (4.01); HMRS: calcd. for C14H12O2N2F7• [M+] 373.0782; found 373.0783.
2-(4-Nitro-2,3,5,6-tetrafluorophenyl)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazol-3-oxide-1-oxyle (2). Yield 200 mg (57%); brown crystals; IR (KBr) ṽmax, cm−1: 417, 446, 476, 538, 608, 712, 768, 785, 804, 874, 972, 999, 1011, 1086, 1142, 1176, 1217, 1265, 1356, 1375, 1389, 1429, 1456, 1486, 1551, 1576, 1626, 2413, 2858, 2931, 2947, 3001, 3442; UV-vis (EtOH) max/nm (lg ): 555 (2.70), 373 (3.5), 318 (4.13), 204 (4.08); HMRS: calcd. for C13H12O4N3F4• [M+] 350.0759; found 350.0758.
2-(2,3,5,6-Tetrafluoropyridin-4-yl)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazol-3-oxide-1-oxyle (3). Yield 83 mg (27%); violet crystals; IR (KBr) ṽmax, cm−1: 453, 538, 567, 646, 696, 715, 868, 958, 972, 993, 1018, 1136, 1169, 1252, 1271, 1375, 1427, 1450, 1475, 1487, 1547, 1655, 1849, 2993, 3437; UV-vis (EtOH) max/nm (lg ): 551 (2.64), 371 (3.89), 288 (4.04), 202 (3.86); HMRS: calcd. for C12H12O2N3F4• [M+] 306.0860; found 306.0865.
2-(Perfluorobiphenyl-4-yl)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazol-3-oxide-1-oxyle (4). Yield 24 mg (5%); violet crystals; IR (KBr) ṽmax, cm−1: 540, 706, 733, 870, 964, 984, 997, 1043, 1134, 1173, 1223, 1267, 1377, 1429, 1487, 1508, 1527, 1595, 1659, 2943, 2995, 3442; UV-Vis (C2H5OH), λmax/nm (lg ε): 549 (2.83), 370 (3.77), 281 (4.10), 237 (4.15), 202 (4.23); HMRS: calcd. for C19H12F9N2O2• [M+] 471,0750; found 471,0747.
3. Crystallographic analysis
XRD experiments for the crystals were carried out on a Bruker Kappa Apex II CCD diffractometer using φ, ω scans of narrow (0.5°) frames with Mo Kα radiation (λ = 0.71073 Å) and a graphite monochromator at 296 K. All the structures were solved by direct methods and refined by the full-matrix least-squares method against all F2 in anisotropic approximation using the SHELX-97 software suite [14]. The positions of H atoms were calculated via the riding model. Absorption corrections were applied empirically in SADABS software applications [15]. To exclude the contribution to the diffraction from the disordered solvent in crystals of 4 and thereby produced a set of solvent-free diffraction intensities, the PLATON/SQUEEZE [16] procedure was employed.
Crystallographic data on all the compounds are listed in Table 3. CCDC 1899824 (1), 1899825 (2), 1899826 (3), and 1899827 (4) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Table 3. Crystal data, data collection and refinement details of nitronyl nitroxides 1–4.
|
1 |
2 |
3 |
4 |
|
|
Crystal data |
||||
|
Chemical formula |
C14H12F7N2O2 |
C13H12F4N3O4 |
C12H12F4N3O2 |
C19H12F9N2O2 |
|
Mr |
373.26 |
350.26 |
306.25 |
471.31 |
|
Crystal system |
Orthorhombic |
Orthorhombic |
Monoclinic |
Tetragonal, |
|
Space group |
Pna21 |
Ibca |
C2/c |
P ̶ 421c |
|
a, (Å) |
8.215(2) |
10.3733(5) |
14.0067(7) |
19.9841(7) |
|
b |
17.781(4) |
11.0785(7) |
10.7414(7) |
19.9841(7) |
|
c |
10.996(2) |
25.589(2) |
10.5168(8) |
10.0603(4) |
|
β (°) |
90 |
90 |
122.419(4) |
90 |
|
V (Å3) |
1606.1(5) |
2940.7(3) |
1335.7(2) |
4017.7 (3) |
|
Z |
4 |
8 |
4 |
8 |
|
F(000) |
756 |
1432 |
628 |
1896 |
|
Dx (Mg m-3) |
1.544 |
1.582 |
1.523 |
1.558 |
|
Radiation type |
Mo Kα |
Mo Kα |
Mo Kα |
Mo Kα |
|
μ (mm-1) |
0.16 |
0.15 |
0.14 |
0.16 |
|
Crystal size (mm) |
0.48 × 0.23 × 0.04 |
0.65 × 0.24 × 0.04 |
0.40 × 0.20 × 0.08 |
0.90 × 0.37 × 0.09 |
|
Data collection |
||||
|
Tmin, Tmax |
0.684, 0.862 |
0.786, 0.862 |
0.805, 0.862 |
0.776, 0.862 |
|
No. of measured, |
25890, |
10284, |
12295, |
43866, |
|
Rint |
0.126 |
0.050 |
0.054 |
0.049 |
|
θ range (°) |
2.2 ̶ 25.1 |
1.6 ̶ 25.0 |
2.6 ̶ 27.5 |
1.4 ̶ 25.1 |
|
Range of h, k, l |
h = -9→ 9, k = -21→ 21, l = -12→ 13 |
h = -12→ 12, k = -13→ 13, l = -30→ 30 |
h = -18→ 18, k = -13→ 13, l = -13→ 13 |
h = -23→ 23, k = -23→ 23, l = -11→ 11 |
|
Refinement |
||||
|
R[F2 > 2σ(F2)], wR(F2), S |
0.072, 0.210, 1.04 |
0.033, 0.135, 1.14 |
0.046, 0.132, 1.03 |
0.050, 0.142, 1.09 |
|
No. of reflections |
2846 |
1305 |
1535 |
3555 |
|
No. of parameters |
229 |
113 |
99 |
293 |
|
No. of restraints |
1 |
0 |
0 |
0 |
|
Δ>max, Δ>min (e Å-3) |
0.34, -0.32 |
0.31, -0.31 |
0.28, -0.30 |
0.24, -0.26 |
Acknowledgments
This work has been supported by the Russian Ministry of Science and Education within the framework of the 5-100 Excellence Programme. The authors thank the Russian Foundation for Basic Research (project 18-33-00203). Authors would like to acknowledge the Multi-Access Chemical Research Center SB RAS for spectral and analytical measurements.
References
- (a) O. Kahn, Molecular Magnetism, Wiley-VCH, New York, 1993; (b) Molecular Magnetism, Eds. K. Itoh and M. Kinoshita, Kodansha & Gordon and Breach, Tokyo, 2000; (c) Magnetism: Molecules to Materials II–IV, Eds. J. S. Miller and M. Drillon, Wiley-VCH, Weinheim, 2001–2005; (d) p-Electron Magnetism From Molecules to Magnetic Materials, Ed. J. Veciana, Springer, Berlin, 2001; (e) Molecular Nanomagnets, Eds. D. Gatteschi, R. Sessoli, and J. Villain, Oxford University Press, New York, 2006; (f) Stable Radicals: Fundamentals and Applied Aspects of Odd Electron Compounds, Ed. R. G. Hicks, Wiley, Chichester, 2010; (g) Y. Morita, S. Suzuki, K. Sato, T. Takui, Synthetic Organic Spin Chemistry for Structurally Well-defined Open-shell Graphene Fragments, Nat. Chem. 3 (2011) 197–204.
- (a) K. Matsuda, M. Irie, A Diarylethene with Two Nitronyl Nitroxides: Photoswitching of Intramolecular Magnetic Interaction, J. Am. Chem. Soc. 122 (2000) 7195–7201; (b) K. Matsuda, M. Irie, Photoswitching of Magnetic Interaction: Diarylethene Photochromic Spin Couplers, Polyhedron 20 (2001) 1391–1395; (c) A. Ito, Y. Nakano, T. Kato, K. Tanaka, Tetraarylethylene Having Two Nitroxide Groups: Redox-switching of Through-Bond Magnetic Interaction by Conformation Change, Chem Commun. (2005) 403–405.
- (a) M. M. Matsushita, H. Kawakami, Y. Kawada, T. Sugawara, Negative Magneto-resistance Observed on an Ion-radical Salt of a TTF-based Spin-polarized Donor, Chem. Lett. 36 (2007) 110–111; (b) M. M. Matsushita, H. Kawakami, T. Sugawara, M. Ogata, Molecule-based system with coexisting conductivity and magnetism and without magnetic inorganic ions, Phys. Rev. B: Condens. Matter Mater. Phys. 77 (2008) 195208; (c) T. Sugawara, H. Komatsu, K. Suzuki, Interplay between Magnetism and Conductivity Derived from Spin-polarized Donor Radicals, Chem. Soc. Rev. 40 (2011) 3105–3118.
- M. Slota, A. Keerthi, W. K. Myers, E. Tretyakov, M. Baumgarten, A. Ardavan, H. Sadeghi, C. J. Lambert, A. Narita, K. Müllen, L. Bogani, Magnetic Edge States and Coherent Manipulation of Graphene Nanoribbons, Nature 557 (2018) 691–695.
- (a) J. H. Osiecki, E. F. Ullman, Studies of Free Radicals. I. α-Nitronyl nitroxides, a New Class of Stable Radicals, J. Am. Chem. Soc. 90 (1968) 1078–1079; (b) E. F. Ullman, J. H. Osiecki, D. G. B. Boocock, R. Darcy, Stable Free Radicals. X. Nitronyl Nitroxide Monoradicals and Biradicals as Possible Small Molecule Spin Labels, J. Am. Chem. Soc. 94 (1972) 7049–7059.
- (a) Y. Hosokoshi, M. Tamura, H. Sawa, R. Kato, M. Kinoshita, Two-dimensional Ferromagnetic Intermolecular Interactions in Crystals of the p-Cyanophenyl Nitronyl Nitroxide Radical, J. Mater. Chem. 5 (1995) 41–46; (b) O. V. Koreneva, G. V. Romanenko, Y. G. Shvedenkov, V. N. Ikorskii, V. I. Ovcharenko, Molecular Magnets Based on M(hfac)2 and Spin-labeled Nitrile, Polyhedron 22 (2003) 2487–2497.
- E. V. Tretyakov, V. I. Ovcharenko, The Chemistry of Nitroxide Radicals in the Molecular Design of Magnets, Russ. Chem. Rev. 78 (2009) 971–1012.
- S. Suzuki, F. Nakamuraa, T. Naota, Direct Synthetic Method for (Nitronyl Nitroxide)-substituted π- Electronic Compounds by Palladium-catalyzed Cross-Coupling Reaction with a Zinc Complex, Mater. Chem. Front. 2 (2018) 591–596.
- (a) M. V. Varaksin, E. V. Tretyakov, I. A. Utepova, G. V. Romanenko, A. S. Bogomyakov, D. V. Stass, R. Z. Sagdeev, V. I. Ovcharenko, O. N. Chupakhin, Synthesis of nitroxyl radical by direct nucleophilic functionalization of a C-H bond in the azadiene systems, Russ. Chem. Bull. 61 (2012) 1469–1473; (b) E. V. Tretyakov, I. A. Utepova, M. V. Varaksin, S. E. Tolstikov, G. V. Romanenko, A. S. Bogomyakov, D. V. Stass, V. I. Ovcharenko, O. N. Chupakhin, New Approach to Synthesis of Nitronyl and Imino Nitroxides Based on SNH Methodology, ARKIVOC viii (2011) 76–98; (c) E. V. Tretyakov, S. E. Tolstikov, G. V. Romanenko, A. S. Bogomyakov, V. K. Cherkasov, D. V. Stass, V. I. Ovcharenko, A Novel Route to Spinlabeled Dihydrooxepines and o-Benzoquinones, Russ. Chem. Bull. 60 (2011) 2325–2330; (d) S. Suzuki, T. Furui, M. Kuratsu, M. Kozaki, D. Shiomi, K. Sato, T. Takui, K. Okada, Nitroxide-Substituted Nitronyl Nitroxide and Iminonitroxide, J. Am. Chem. Soc. 132 (2010) 15908–15910.
- E. V. Tretyakov, P. A. Fedyushim, E. V. Panteleeva, D. V. Stass, I. Y. Bagryanskaya, I. V. Beregovaya, A. S. Bogomyakov, Substitution of a Fluorine Atom in Perfluorobenzonitrile by a Lithiated Nitronyl Nitroxide, J. Org. Chem. 82 (2017) 4179–4185.
- (a) P. Fedyushin, E. Panteleeva, I. Bagryanskaya, K. Maryunina, K. Inoue, D. Stass, E. Tretyakov, An approach to fluorinated phthalonitriles containing a nitronyl nitroxide or iminonitroxide moiety, J. Fluor. Chem. 217 (2019) 1–7; (b) O. N. Chupakhin, I. A. Utepova, M. V. Varaksin, E. V. Tretyakov, G. V. Romanenko, D. V. Stass, V. I. Ovcharenko, SNH Approach in the Synthesis of Nitronyl Nitroxides, J. Org. Chem. 74 (2009) 2870–2872.
- L. V. Politanskaya, G. A. Selivanova, E. V. Panteleeva, E. V. Tret'yakov, V. E. Platonov, P. V. Nikul'shin, A. S. Vinogradov, Y. V. Zonov, V. M. Karpov, T. V. Mezhenkova, A. V. Vasil'ev, A. B. Koldobskij, O. S. Shilova, M. V. Morozova, Y. V. Burgart, E. V. Shchegol'kov, V. I. Saloutin, V. B. Sokolov, A. Yu. Aksinenko, V. G. Nenajdenko, M. Yu. Moskalik, V. V. Astahova, B. A. Shainyan, A. A. Tabolin, S. L. Ioffe, V. M. Muzalevskij, E. S. Balenkova, A. V. Shastin, A. A. Tyutyunov, V. E. Bojko, S. M. Igumnov, A. D. Dil'man, N. Y. Adonin, V. V. Bardin, S. M. Masoud, D. V. Vorob'eva, S. N. Osipov, E. V. Nosova, G. N. Lipunova, V. N. Charushin, D. O. Prima, A. G. Makarov, A. V. Zibarev, B. A. Trofimov, L. N. Sobenina, K. V. Belyaeva, V. YA. Sosnovskih, D. L. Obydennov, S. A. Usachev, Sovremennaya ftororganicheskaya himiya v Rossii, Uspekhi himii 88 (2019) in press; DOI: 10.1070/RCR4871.
- F. H. Allen, O. Kenard, D. G. Watson, L. Bramer, A. G. Orpen, R. Taylor, Tables of Bond Lengths determined by X-Ray and Neutron Diffraction. Part I. Bond Lengths in Organic Compounds, J. Chem. Soc. Perkin Trans. II 12 (1987) s1–s7.
- G. M. Sheldrick, SHELX-97, Programs for Crystal Structure Analysis (Release 97-2), University of Göttingen, Germany, 1997.
- SADABS, v. 2008-1, Bruker, AXS, Madison, WI, USA, 2008.
- (a) A. L. Spek, PLATON, A Multipurpose Crystallographic Tool (Version 10M), Utrecht University, The Netherlands, 2003; (b) A. L. Spek, Single-crystal Structure Validation with the Program PLATON, J. Appl. Crystallogr. 36 (2003) 7–13.
Supplementary Materials with data of IR and UV spectra see in PDF version.
Recommended for publication by Prof. V.G. Nenaidenko
Fluorine Notes, 2019, 123, 7-8
