Received: February 2019
DOI 10.17677/fn20714807.2019.02.01
Fluorine Notes, 2019, 123, 1-2
Synthesis and Repellent Properties of Fluorinated Diblock-Copolymers
K.E.Chekurov*, A.I. Barabanova, I.V. Blagodatskhikh, A.S. Peregudov, A.R. Khokhlov
A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences,28 Vavilov Street, GSP-1, Moscow, Russian Federation
kirillswim@rambler.ru
Abstract: The radical polymerization of 2,3,4,5,6-pentafluorostyrene (PFS) and 2-hydroxyethyl methacrylate (HEMA) in the presence of chain transfer agent - 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid was studied. It was shown that PFS polymerization proceeds through a reversible addition-fragmentation chain transfer polymerization mechanism, and that of HEMA polymerization is more complicated. The conditions of preparation of diblock copolymers (DBC) which do not contained the by-products of uncontrolled radical polymerization of PFS were found. Comparative study of contact angles versus water (θН2О) and diiodomethane (θCH2I2) of nylon fabric samples, treated with DBC containing 37 mol. % PFS-units, and poly-2,3,4,5,6-pentafluorostyrene (PPFS) was conducted. It is shown that the nylon fabric treated with DBC has higher θН2О = 125±3° and θCH2I2 = 101±5° than PPFS films (θН2О = 102±2° иθCH2I2 = 74±1°).
Key words: 2,3,4,5,6-pentafluorostyrene, amphiphilic diblock copolymers, repellent properties
Introduction
To create omniphobic coatings, compounds containing long-chain fluorinated alkyl fragments with terminal -CF3 groups, which are capable of self-organization with dense packing of -CF3 groups, are commonly used. Dense packaging provides low values of the surface energy of the coatings and gives them the ability to not be wetted by both polar and non-polar liquids. However, long fluorinated fragments do not decompose in nature, and the destruction of coatings is accompanied by the release of toxic and bioaccumulative perfluorooctane sulfonic and perfluorooctanoic acids. In this regard, the urgent task is the creation of omniphobic coatings without the use of compounds with long-chain fluorinated alkyl fragments.
In this work, we propose to use amphiphilic diblock copolymers (DBC) consisting of fluorine-containing and hydroxyl-containing polymer blocks to create omniphobic coatings. Amphiphilic DBC as a result of microphase separation and effective self-organization are able to create dense packing of fluorine-containing polymer blocks on substrates and impart repellent properties to coatings, while hydroxyl-containing polymer blocks ensure crosslinking of DBC with porous substrates [1-3].
The aim of the work is to obtain fluorine-containing DBC by two-stage radical polymerization with reversible chain transfer by the addition-fragmentation mechanism (RAFT-polymerization) of 2,3,4,5,6-pentafluorstyrene (PFS) with 2-hydroxyethyl methacrylate (HEMA) in the presence of a chain transfer agent (CTA) - 4-cyano-4- (thiobenzoylthio) pentanoic acid (CPDTB), and study of the repellent properties of coatings from synthesized DCB.
Experimental part
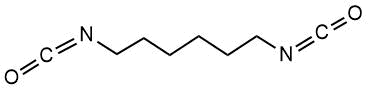
We used PFS monomers (PIM-INVEST, Russia) and HEMA (98% ZL Chemical, China). PFS and HEMA were distilled under vacuum before use (P = 0.1 mbar, T = 63°C and 105°C for PFS and HEMA, respectively). CPDTB (> 97%, Sigma-Aldrich, Germany) was used as a CTA without prior purification. The polymerization initiator α-bis-isobutyric acid dinitrile (AIBN) (98%, Sigma-Aldrich, Germany) was recrystallized twice from methanol. Hexamethylene diisocyanate (HMDI) (> 99%, Sigma-Aldrich, Germany) was used as a crosslinker for polymer films with a nylon fabric surface. The solvents used were purified according to conventional techniques.
Poly-2,3,4,5,6-pentafluorostyrene (PPFS-CTA) and poly-2-hydroxyethyl methacrylate (PHEMA-CTA) were synthesized by RAFT-polymerization of the corresponding monomers in DMF at 60 °C in Schlenk tubes with a volume of 15-20 cm3 in vacuum (residual pressure 0.1 mbar) in the presence of AIBN and CPDTB. RAFT-polymerization of PFS ([PFS] = 2.0 mol / l) was carried out at a molar ratio of [CPDTB] / [AIBN] = 1.9 and initiator concentration [AIBN] = 2.7 × 10-3 mol/l. PHEMA-CTA’s were synthesized by polymerization of HEMA ([HEMA] = 2 mol / l) with [CPDTB] = 2 × 10-2 mol / l and [AIBN] = 8 × 10-3 mol / l. Before polymerization, the reaction mixture was freed from air by repeated repetition of freeze-thaw cycles in vacuum, then transfered the Schlenk tube to a thermostat heated to 60 °C. The resulting PPFS-CTA’s were isolated from the reaction mixture by twice precipitation in excess of methanol, PHEMA-CTA’s were precipitated in excess of chloroform, followed by centrifugation for 10 min at 11000 rpm (Rotina R38, Germany). The isolated polymers were dried at room temperature under vacuum to constant weight. Conversion was determined gravimetrically.
DBC’s synthesized by RAFT polymerization of PFS in the presence of the PHEMA-CTA’s. RAFT polymerization of PFS ([PFS] = 2 mol/l) carried out at 60°С to DMF under the influence of thermal disintegration of AIBN ([AIBN] = 1 × 10-3 mol/l) at [PHEMA] / [AIBN] = 2 and 5. Upon termination of reaction the product was precipitated at 10-15 multiple excess of chloroform with the subsequent centrifugation within 10 min. at 11000 rpm (Rotina R38, Germany) and dried in a vacuum to constant weight. The composition of DBC was determined by the element analysis. Conversion was estimated gravimetrically.
Chemical formulas of monomers, CTA, m-CTA and initiator for the synthesis of DBC, and a crosslinker for the preparation of polymer coatings on fabrics.
|
Monomers |
HEMA |
 |
|
PFS |
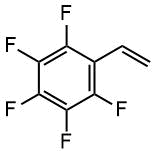 |
|
|
CTA |
CPDTB |
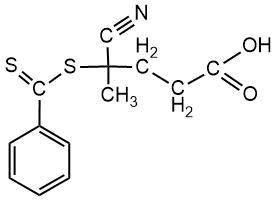 |
|
Initiator |
AIBN |
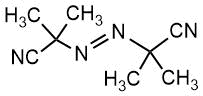 |
|
m-CTA |
P(HEMA)-CTA |
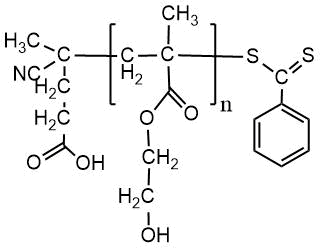 |
|
Crosslinker |
HMDI |
 |
Molecular-mass (MM) characteristics (Mn, Mw and Mw/Mn) Molecular-mass (MM) characteristics (Mn, Mw and Mw/Mn) determined by gel-penetrating chromatography (GPC) on the liquid Agilent 1200 chromatograph supplied with the refractometric detector in the GPC mode and the system of recording and data processing of ChemStation 1200 (Agilent). For the analysis of PPFS used the column PLmixC (Agilent) in THF at a temperature of 25°C and the flow rate of 1 ml/min. For the analysis of PHEMA and DBC used the G-gel column (a spherical macroporous sorbent on the basis of the hydrolyzed glycidylmethacrylate copolymer with ethylendimethacrilate [4, 5]) at a temperature of 30°C and flow rate of eluent of 0.5 ml/min. As eluent for PHEMA used 0.03 M LiBr in DMF, for the analysis of DBC and comparison with an initial mCTA by used mix THF: 0.03 M LiBr in DMF (50: 50 about. %). Calibration of the device was carried out according to the polystyrene Waters and Merk standards.
The PMR spectra were recorded on an AvanceTM 600 spectrometer by Bruker (Germany), the proton operating frequency of 600.22 MHz. Chemical shifts are determined relative to the residual signal of CHCl3 in the deuterium solvent (7.28 ppm) and are converted to TMS. Accuracy of determination of chemical shifts is not worse than 0.001 ppm.
Films from PPFS and PHEMA were prepared by immersing nylon fabric samples (1.5 cm × 1.5 cm) in PPFS solutions in THF or PHEMA in DMF (solution volume 2 ml, polymer concentration 50 mg / ml) for 3 hours, followed by drying at room temperature for 6 h and annealing in a vacuum oven (LabTech, Russia, Pres = 6 × 10-2 Pa) at 110 °C for 1 h.
Films from DBC were applied on nylon fabric in the presence of HMDI. Concentration of HMDI was calculated, believing what each OH-group of HEMA from PHEMA-block contacts the surface of fabric. Films were prepared by the technique described in [1]. Samples of nylon fabric (1.5 cm × 1.5 cm) immersed in 2 ml of DBC solution in DMF with HMDI ([DBC] = 50 mg/ml, [HMDI] = 45 mg/ml) for 3 hours, then dried at room temperature for 6 h with followed annealing in a vacuum oven (LabTech, Russia, Pres = 6 × 10-2 Pa) at 110 °C during 1 hour.
The repellent properties of the films on the surface of the nylon fabric were determined by static edge wetting angle with water (θН2О) and diiodomethane (θCH2I2) using the sitting drop method KrussDSA25 (Germany), accuracy 1°.
Results and discussion
RAFT polymerization of PFS in the presence of CPDTB
There are not many works dedicated on the RAFT-polymerization of the PFS [1,6,7]. It was previously shown that the polymerization of PFS according to the RAFT mechanism of polymerization proceeds in the presence of 2-cyano-2-propyl-dithiobenzoate (CPDB) [1] and 2-(dodecylthiocarbonothioylthio)-2-methylpropionic acid (DDMAT) [6,7]. Since the controlled nature of the RAFT polymerization is determined largely by the nature of the CTA, the search for the most effective CTA is constantly ongoing. In the present work, the polymerization of PFS was studied for the first time in the presence of CPDTB and AIBN in DMF. The conditions of polymerization and MM characteristics of the synthesized PPАS are shown in Tab. 1.
Figure 1 shows the PMR spectra of PPFS synthesized by radical polymerization (Fig. 1, 1) and RAFT polymerization (Fig. 1, 2), as well as the CPDTB (Fig. 1, 3). Signals at 2.05, 2.35 and 2.8 ppm refer to the protons –CH- and –CH2-groups of the main polymer chain in PPFS (Fig. 1, 1 and 2). In the PMR spectrum of the CPDTB, signals of the protons of the phenyl group are observed, manifested as a doublet at 7.94 ppm (two ortho-protons), a pseudotriplet at 7.42 ppm. (two meta-protons) and triplet at 7.59 ppm (one para-proton). In the PMR spectrum of PPFS synthesized by the RAFT polymerization, broadened signals of the phenyl protons of the stabilizing group of the CPDTB is appear. The inset shows an enlarged fragment of the spectrum with an ortho-proton signal at 7.96 ppm, which is absent in the PMR spectrum of PPFS synthesized by free radical polymerization. Multiplets in the region of 2.82 - 2.43 ppm referring to the four methyl protons –CH2, the singlet at 1.97 ppm to the three protons –CH3 of the leaving group of the CPDTB, in the PMR spectrum of the PPFS (Fig. 1, 2), is overlapped by the –CH2-groups of the main polymer chain in the PPFS and therefore not separately detected.
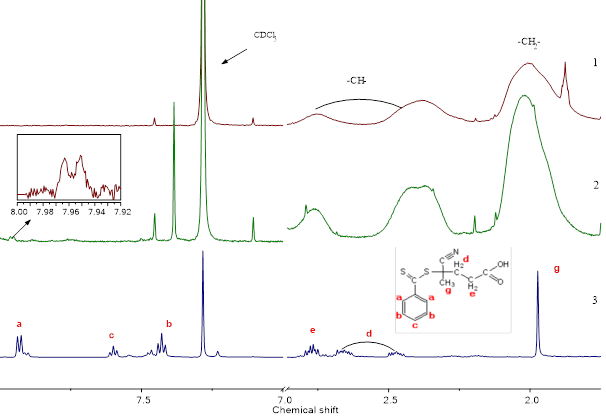
Fig. 1. Spectrum PMR PPFS, obtained by radical (1) and RAFT polymerization (2), and
CPDTB (3) in CDCl3.
From the ratio of the integral intensities of proton signals –CH groups of the main polymer chain at 2.35 and 2.8 ppm and signals of the ortho-protons of the phenyl group of the mCTA at 7.96 ppm in the PMR PMPR spectra (Fig. 1, 2) synthesized by the RAFT polymerization, the Mn value was determined by equation (1)
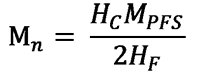 (1)
(1)
where HC and HF are the integral intensities of the –CH-group signals of the main polymer chain of PPFS and the signals of the ortho-protons of the phenyl group of the CTA, the MPFS is the molecular weight of the PFS. In this case, it was assumed that all polymer chains contain terminal phenyl groups of CPDTB.
In Fig. 2 shows the MWD curves for PPFS obtained by polymerizing PFS under the action of the thermal decomposition of AIBN in the presence of the CPDTB at 60 °C for different periods of time. For all PPFS, the MWD curves are symmetric and unimodal and, with increasing conversion, are successively shifted toward higher molecular weights. From Table 1 it can be seen that the values of Mn (PMR) and Mn (GPC) are in satisfactory agreement with each other, although Mn (GPC) are not absolute values, but are calculated relative to polystyrene.
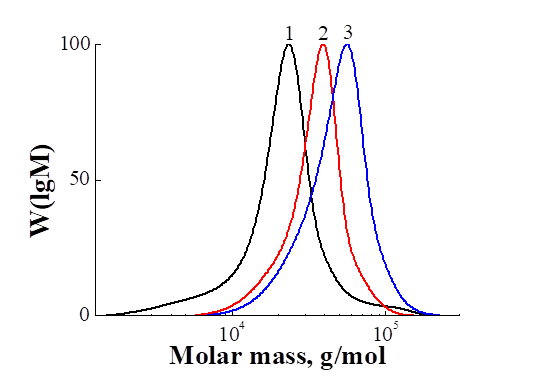
Fig. 2. Comparison of the MWD curves of PPFS1, PPFS2, and PPFS3.
The dependences of theoretical and experimental Mn synthesized PPFS on the monomer conversion are presented in Tab. 1 and Fig. 3. Theoretical values of Mn (theor) were calculated, assuming that one molecule of a CPDTB leads to the growth of a single polymer chain, according to equation (2):
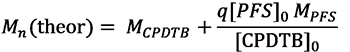 (2)
(2)
where MCPDTB and MPFS – molecular masses of the CTA and PFS, [CPDTB]0 and [PFS]0 – their initial concentration, q – conversion of PFS.
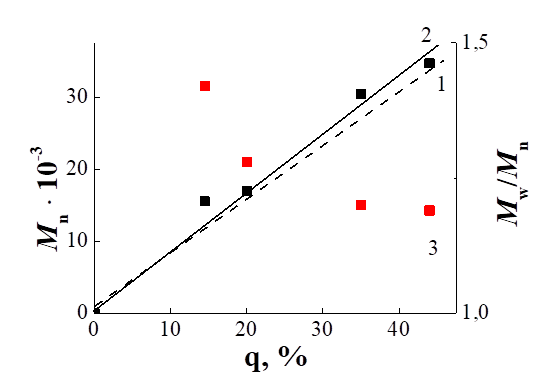
Fig. 3. Dependences of Mn (theor) (1), Mn (2) and Mw / Mn (3) on the conversion of PFS during polymerization in DMF. [PFS] = 2.0 mol / l, [AIBN]
= 2.7 × 10-3 mol / l, [CPDTB] = 5.8 × 10-3 mol / l, T = 60°C.
Table 1. Polymerization of PFS and MM characterictics of P(PFS)
|
№№ |
Time, h |
q, % |
Mn× 10-3 |
Mw/Mn |
||
|
theor |
NMR |
GPC |
||||
|
PPFS1 |
10 |
14.5 |
10 |
24 |
15.5 |
1.42 |
|
PPFS2 |
14 |
20 |
13.7 |
26.5. |
17.0 |
1.28 |
|
PPFS3 |
18 |
35 |
23.7 |
35 |
30.5 |
1.20 |
|
PPFS4 |
24 |
44 |
29.7 |
36.9 |
33.0 |
1.19 |
*[CPDTB] / [AIBN]=1.9; [PFS]= 2 моль/л; [AIBN] = 2.7 × 10-3 mol/l; 60°С; DMF
It can be seen that the experimental values of Mn (GPC) PPFS are close to the theoretical values of Mn (theor) and linearly increase with conversion. Higher experimental values of Mn (PMR) at q <20% compared with both Mn (theor) and Mn (GPC) are most likely due to the incomplete expenditure of the CTA. The polydispersity coefficients of all PPFS are much lower than in the classical radical polymerization, and decrease with a monomer conversion from 1.42 to 1.19 (Table 1). The observed change in Mn and Mw / Mn with PFS conversion indicates the pseudo-living nature of the PFS polymerization reaction in the presence of the CPDTB.
Thus, it was established that the polymerization of PFS in the presence of a CPDTB proceeds according to the RAFT-polymerization mechanism with the formation of narrowly dispersed polymers with a sufficiently high MM.
RAFT polymerization of HEMA in the presence of CPDTB
From the literature it is known that the HEMA polymerized by RAFT-mechanism in the presence of CPDB [1, 8] cumil dithiobenzoate [9], 4-cyano-4-(butylsulfanylthiocarbonyl) sulfanyl pentanoic acid and ethylene glycol di((1-butyl)sulfanylthiocarbonylsulfanyl-4-cyanopentanoate) [10]. In this paper, we first performed the polymerization of HEMA in the presence of a CPDTB. In Tab. 2 shows the conditions for the synthesis and MM characteristics of PHEMA. In Fig. 4 shows the MWD curves for PHEMA synthesized over different periods of time. It can be seen that all the MWD curves are unimodal and with increasing and shifting towards higher masses. The linear growth of Mn from 21,400 to 30,700 and low values of the polydispersity coefficient (1.25 - 1.20) indicate the pseudo-living nature of the polymerization (Fig. 5), however, the observed mismatch of Mn (theor) and Mn (GPC) (Table 2) indicates that that the mechanism of the polymerization reaction is possibly more complex.
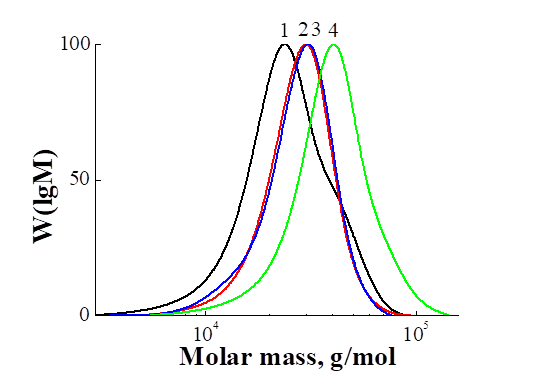
Fig. 4. Comparison of MWD curves of PHEMA 1, PHEMA 2, PHEMA 3 and PHEMA 4.
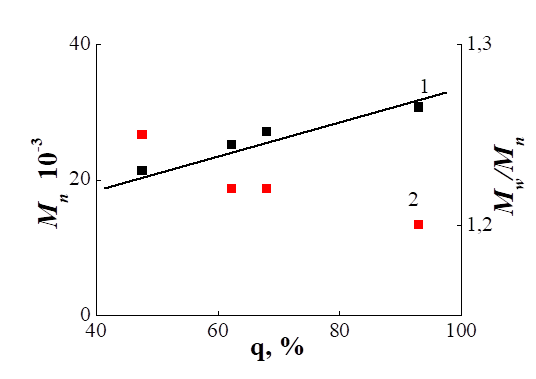
Fig. 5. Dependences of Mn (1) and Mw / Mn (2) on HEMA
conversion during polymerization in DMF. [HEMA] = 2.0 mol / l, [AIBN] = 8.0 × 10-3 mol
/ l, [CPDTB] = 2.0 × 10-2 mol / l, T = 60°C.
Table 2. Polymerization of HEMA and MM characterictics of P(HEMA)
|
№№ |
Time, h |
q, % |
Mn× 10-3 |
Mw/Mn |
|
|
theor |
GPC |
||||
|
PHEMA1 |
6 |
47 |
6.4 |
21.4 |
1.25 |
|
PHEMA2 |
8 |
62 |
8.4 |
25.2 |
1.22 |
|
PHEMA3 |
10 |
68 |
9.2 |
27.1 |
1.22 |
|
PHEMA4 |
18 |
93 |
12.4 |
30.7 |
1.20 |
*[CPDTB] / [AIBN]=2.5; [HEMA]= 2 моль/л; [AIBN] = 8 × 10-3 mol/l; 60°С; DMF
Synthesis of DBC
DBC can be synthesized by polymerization of HEMA in the presence of PPFS-CTA's or by polymerization of PFS in the presence of PHEMA-CTA's. Our experiments showed that the polymerization of HEMA, initiated by AIBN, is inhibited in the presence of PPFS-CTA. The inability of the PPFS-CTA to re-initiate the polymerization of HEMA is probably due to the fragmentation of PPFS intermediates [11]. It should be noted that amphiphilic DBC PFS with HEMA [1], with methacrylate derivatives, 4-hydroxy styrene and 4-vinylpyridine [7], as well as with glycidyl methacrylate [12], were obtained starting from hydrophilic blocks and not from PPFS blocks.
Polymerization of PFS in the presence of the PHEMA-CTA's
The scheme of the two-stage synthesis of DBC by polymerizing PFS in the presence of a PHEMA-CTA with a dithiobenzoate terminal group is shown below.
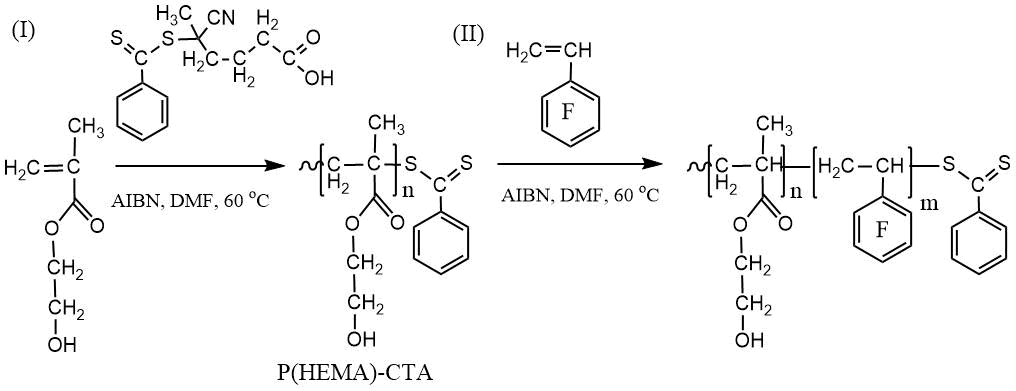
Scheme of synthesis of PHEMA-CTA (I) and DBC (II) by RAFT polymerization
Conditions for obtaining DBC and their MM characteristics are collected in Table 3.
Table 3. Synthesis of DBC.
|
№№ |
[PHEMA4]/[AIBN] |
Time, h |
q, % |
GPC |
Composition of DBC, mol. % |
||
|
Mn× 10-3 |
Mw/Mn |
HEMA |
PFS |
||||
|
DBC1 |
2 |
18 |
17 |
34.7 |
1.53 |
50 |
50 |
|
DBC2 |
2 |
24 |
27 |
61.6 |
1.40 |
35 |
65 |
|
DBC3 |
5 |
18 |
38 |
43.9 |
1.29 |
63 |
37 |
*[PFS] = 2 mol/l; [AIBN] = 1 × 10-3 mol/l; 60°С; DMF
A comparison of the MWD curves for PHEMA4 and the products of polymerization of the PFS is shown in Fig. 6. On the MWD curves of PFS polymerization products with [PHEMA4] / [AIBN] = 2 for 18 and 24 hours, one can see a shoulder (Fig. 6, curves 3 and 4), the appearance of which may be due to the formation of PPFS as a result of radical polymerization, moreover, with an increase in the duration of the reaction, both the main peak and the shoulder of the MWD curves shift towards higher molecular weights. The polydispersity coefficients calculated from these curves exceed the values characteristic of RAFT polymerization. The MWD curve for DBC3, synthesized with [PHEMA4] / [AIBN] = 5, is unimodal, symmetrical and shifts from the original PHEMA4 towards higher molecular weights, which indicates that PFS polymerization proceeds through the RAFT polymerization without the formation of radical polymerization by-products of PFS (Fig. 6, curve 2). From Tab. 3 it follows that DBC3 is characterized by a low polydispersity characteristic of pseudo-living polymerization products Mw / Mn = 1.29.
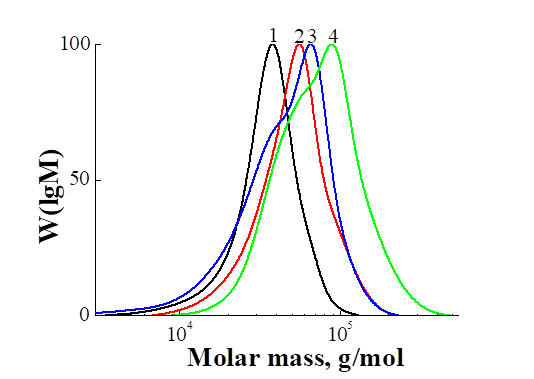
Fig. 6. Comparison of the MWD curves of PHEMA4 (1) and the products of polymerization
of PFS in the presence of PHEMA4: DBC3 (2), DBC1 (3) and DBC2 (4).
Research of repellent properties of films from PPFS, PHEMA and DBC3
The repellent properties of samples of nylon fabric treated with PPFS, PHEMA and DBC3 are presented in Table. 4. Fabric treated with PHEMA absorbs both water and DI due to capillary effects. After applying PPFS and DS3, the samples become hydrophobic (Fig. 7), with the θН2О value of the fabric treated with DS3 being 125 3°, which is 23° higher than θН2О for the fabric impregnated with PPPS (θН2О = 102 2°) despite that DBC contains only 37 mol. % hydrophobic units of the PFS. Static contact angles of wetting DI (drop volume 1.5 μl) for fabrics treated with PPFS and DBC3 are 80 2° and 101 5°, respectively. The observed improvement in the repellent properties of the surface of DBC3 films compared with the corresponding parameters for PPFS films can be due to both the induced surface curvature and the perpendicular orientation of PPFS blocks to the substrate surface and their dense packing as a result of self-organization of amphiphilic DBC [1-3].
Table 4. Edge angles of the surface of coatings made of PPFS, PHEMA and DBC3 on nylon fabric
|
Sample |
[ОН]/[HMDI], mol/mol |
Fabric |
|
|
H2O |
СH2I2 |
||
|
PPFS |
- |
102±2 |
74±1 |
|
PHEMA |
- |
Absorbed |
Absorbed |
|
DBC3 |
236/1 |
125±3 |
101±5 |
 |
 |
| (a) | (b) |
Fig. 7. Photo image of a water droplet with a volume of 1.5 µl on the surface of a PPFS film (a) DBC3 (b) on a nylon fabric.
Conclusion
The polymerization of PFS and HEMA in the presence of the CPDTB was studied. It is established that the polymerization of PFS proceeds by a pseudo-living radical mechanism. During the polymerization of HEMA in the presence of CPDTB, with an increase in the duration of the reaction, a linear increase in the molecular mass of narrowly dispersed polymers with Mw / Mn = 1.20 - 1.25 is observed. However, the experimental values of Mn (GPC) significantly exceed the theoretical values, which probably indicates a more complex mechanism of the reaction. During the polymerization of PFS in the presence of PHEMA-CTA with [PHEMA4] / [AIBN] = 2, products are formed containing both DBC and a small amount of by-product of PPFS resulting from uncontrolled radical polymerization of PFS. If you increase the content of PHEMA4 to [PHEMA4] / [AIBN] = 5, only DBC is formed. Films of synthesized DBC with a PFS content of less than 40%, chemically bonded to the surface of the nylon fabric, give it repellent properties to both water and diiodomethane, superior to the properties of PPFS films.
The study was carried out at the expense of a grant from the Russian Science Foundation (project No. 17–13–01359) at the Institute of Organoelement Compounds to them. A.N. Nesmeyanov Russian Academy of Sciences (INEOS RAS).
The structure of the compounds obtained was studied using the equipment of the Center for the Study of the Structure of Molecules of the INEOS RAS.
References
- Chekurov K.E., Barabanova A.I., Blagodatskikh I.V., Lokshin B.V., Peregudov A.S., Abramchuk S.S., Khokhlov A.R. // Dokl. AN.2019, 484 (4)(in published).
- Szczepanski C.R., Darmanin T., Guittard F. // ACS Appl. Mater. Interfaces, 2016, 8 (5), 3063–3071.
- Yamaguchi H., Kikuchi M., Kobayashi M., Ogawa H., Masunaga H., Sakata O., Takahara A. //Macromolecules,2012, 45, 1509-1516.
- Tennikova T. B., Horak D., Svec F., Kolar J., Coupek J., Trushin A., Maltzev V.G., Belenki B.G. //J. Chromatigr. A., 1988, 435, 357-362.
- Fomenkov, A.I., Blagodatskikh, I.V., Ponomarev, Iv.I.,Volkova, Yu.A., Ponomarev, I.I., and Khokhlov, A.R.//,Vysokomol. Soed. B, 2009, 51 (5), 874-882.
- Ma J., Cheng C., Sun G., Wooley K.L. //Macromolecules, 2008, 41 (23), 9080-9089.
- Riedel M., Stadermann J., Komber H., Simon F., Voit B.// Eur. Polym. J., 2011, 47 (4), 675-684.
- Sundhoro M., Park J., Jayawardana K.W., Chen X, Jayawardena H.S.N., Yan M. // J. Colloid Interface Sci.,2017, 500, 1-8.
- Kodama Y., Barsbay M., Güven O. // Radiat. Phys. Chem., 2014, 105, 31-38.
- Radzevicius P., T. Krivorotova T.,Makuska R. // Eur. Polym. J., 2017, 87, 69-83.
- Gregory A., Stenzel M.H. // Prog. Polym. Sci., 2012, 37, 38-105.
- Gudipati C.S., Tan M.B.H., Hussain H., Liu Y., He C., Davis T.P. // Macromol. Rapid. Commun., 2008, 29 (23), 1902-1907.
Recommended for publication by Prof. S. M. Igoumnov
Fluorine Notes, 2019, 123, 1-2
