Received: October 2018
DOI 10.17677/fn20714807.2019.01.03
Fluorine Notes, 2019, 122, 5-6
Electrochemical anodic synthesis of Tb3+ luminescent coordination compounds with some fluorophenylacetic acids
M.A. Nazarenko, A.I. Oflidi, S,L. Kuznetsova
Kuban State University, 350040, Stavropolskaya street, 149, Krasnodar, Russia,
e-mail: lantan-100@yandex.ru
Abstract: the results of electrochemical anodic synthesis of coordination compounds of terbium(III) with pentafluorophenylacetic and 2,3,5,6- tetrafluoro-4-(trifluoromethyl)phenylacetic acids are reported. The structure and properties of terbium(III) synthesized coordination compounds were held by the IR- and luminescence spectroscopy. According to IR-spectra data, the bond type of carboxylated ligand’s atom with lanthanid’s ion is mainly ionic. It was found out that at the room temperature terbium (III) complex compounds luminesce in visible area (green luminescence) and have emission bands, corresponding to Tb3 ion energy transitions.
Keywords: electrochemical anodic synthesis, coordination compounds, fluorophenylacetic acids, luminescence.
In recent times [1-4], the development of electroluminescent materials on the base of lanthanides compounds cuts a swath due to the possibility of its wide practical application in various scientific and technical fields. Organic light-emitting diodes (OLEDs) are the base for electroluminescent materials. The production technology of electroluminescent materials involves usage of polymeric, organic or metal-complex compounds which radiate light under the influence of current flow.
Metal nature, its coordination environment and stereochemistry of the metal center exert a significant impact on useful properties of electroluminescent complexes. It is strongly shown in lanthanides complexes because of the lanthanides ions electronic structure features and its initiation through an organic part of complex compound, where the probability of ligand’s energy conversion from singlet state into a triplet state (S1→T1 transition) is high [4].
However, the hydrolysis, hydration, contaminating of target compound by reaction by-products, makes it not always possible to obtain the compounds, that will carry out all the specified requirements, by using classical chemical methods of coordination compounds synthesis. So, the presence of coordinate water, as a part of complex compound, can lead to reduction of luminescence quantum efficiency. The electrochemical anodic synthesis does not have these negative attributes and it allows, by performing synthesis in one stage, to obtain waterless compounds that do not contain foreign ions and also to vary structure of a target product and the direction of synthesis.
Anodic synthesis is a direct synthesis method and it’s idea is concluded in coordination sphere formation through the metals oxidation in zero oxidation level and ligands. The possibility to synthesize those coordination compounds, which are impossible to obtain by other classical methods, and soft synthesis conditions with rather high yields of a target product are the main advantages of this type of synthesis [5].
Electrochemical synthesis of terbium (III) and gadolinium (III) complex compounds with some fluorophenylacetic acids, analysis of its composition and luminescent properties are the aims of this paper.
Electrochemical synthesis. When using electrochemical anodic synthesis methods, for its maximum efficiency, it is important to define the optimal conditions and parameters.
We chose solvent (acetonitrile) because it has such properties as: electrochemical stability, low coordinating capacity, availability, easiness of dehydration and also, parent fluorophenylacetic acids and background electrolyte are soluble in it.
Lithium perchlorate is freely soluble in acetonitrile and its ions have the low coordinating ability, so it was used as background electrolyte during the synthesis.
The proper current force during the syntheses of lanthanides complex compounds with the used ligands is equal to 15 - 20 mA; in order to achieve it, we applied voltage 8 – 10 W on an electrochemical cell. If the current force is lower, then the synthesis process slows down, if the current force is higher, then we can observe the solution heating what can lead to side reactions. Due to this fact, we held the synthesis at the temperature, not higher than 30оС. The optimal anodic current density during the processes varied between 9-11 mA/cm2. In case of higher current density – intensive anode destruction starts, what leads to synthesis efficiency degradation and final product contamination with metal-containing particulate materials.
Slightly soluble coordination connections were formed during the synthesis process, an adhesion of the formed complex happened on the anode, what led to passivation of an electrode. Decrease of the current density almost to zero was the result of passivation and this led to the process deceleration. Due to the high electrical resistance, the system average electrical conductivity also decreased. For the solution of these problems the electrochemical cell during the syntheses was exposed by ultrasonic treatment and thanks to that, there was a considerable decrease in anode passivation and stabilization of synthesis process.
At terbium (III) complex compounds synthesis the yield by metal is equal to ~85%, yield by current is equal to ~80%. The high yields confirm electrochemical anodic synthesis efficiency when obtaining these compounds and when choosing the optimal conditions [6].
IR-spectroscopy. The comparison of IR-spectrum of obtained coordination compounds with IR-spectrum of initials of fluorophenylacetic acids shown that the last in complexes are in ionized form, because there appears absorption bands of asymmetrical and symmetrical oscillations of deprotonated carboxylic group in the field 1650-1510 cm-1 and 1440-1370 cm-1 correspondingly, and there disappears absorption bands in the field 1665-1700 cm-1, which refers to valence vibrations of C=O bond of nonionized carboxy group. The difference between ionized carboxy group asymmetrical and symmetrical oscillations ν(СOO-) is less than 220 cm-1, what allows to assume [7] its bidentate coordination with Tb3+ ion in obtained complex compounds. Due to the same reason, we can assume that ligand’s carboxylated oxygen atom with Tb3+ ion bond type has mainly ionic character.
Luminescence. The studied terbium (III) complex compounds with pentafluorophenylacetic and 2,3,5,6- tetrafluoro-4-(trifluoromethyl)phenylacetic acids luminesce in visible area (green luminescence) at room temperature and have emission bands, corresponding to Tb3+ ion energy transition: 5D4→ 7F6 (490 nm, 20500 cm-1), 5D4→7F5(543 nm, 18400 cm-1), 5D4→ 7F4 (585 nm, 17000 cm-1), 5D4→ 7F3 (620 nm, 16000 cm-1). At the same time, the fact, that there is no organic ligand phosphorescence, may prove good energy redistribution on Tb3+ ion. This is connected with ligands initiated triplet levels optimal location and terbium (III) ion emissive level for the obtained compounds.
Tb3+ compound with pentafluorophenylacetic acid has the highest quantum efficiency of luminescence
(Picture 1).
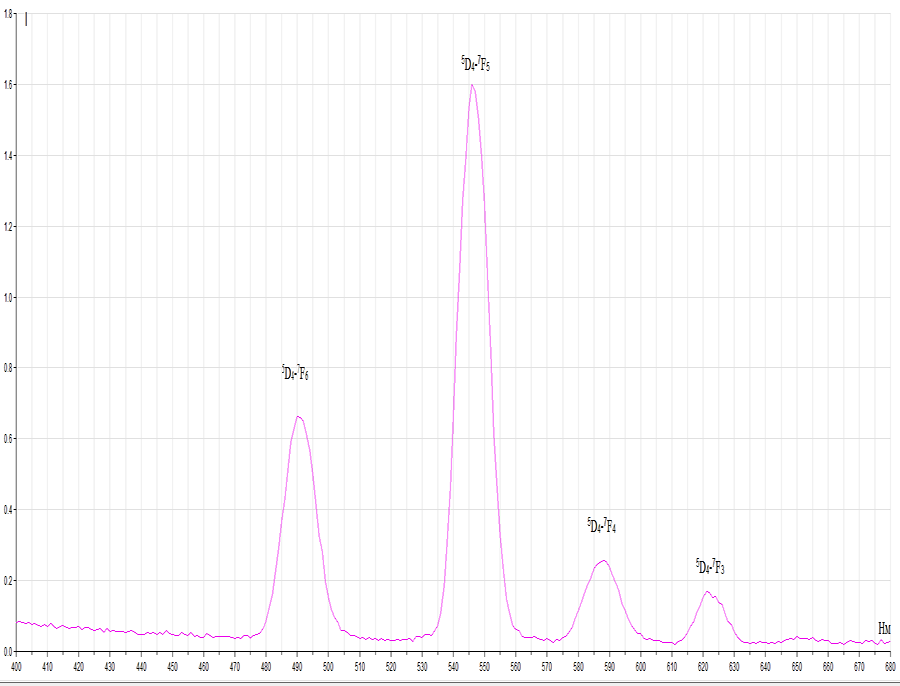
Picture 1. –Tb3+ сcomplex compound with pentafluorophenylacetic acid luminescence spectrum.
Experimental
In the process were used – metal terbium in plates (purity – 99,9%), fluorophenylacetic acids: pentafluorophenylacetic acid chemically pure and 2,3,5,6- tetrafluoro-4-(trifluoromethyl)phenylacetic acid chemically pure.
Direct electrochemical synthesis of complex compounds was held using the method of the soluble anode with the usage of a direct current source in a two-electrode cell.
The cell consists of a glass reactor with densely grinded top in which electrodes – the terbium anode and the platinum cathode are located; on the bottom of a cell the magnetic mixer anchor for constant solution stirring is placed.
The dehydrated acetonitrile was used as nonaqueous solvent at electrochemical synthesis. Time of synthesis was determined, proceeding from initial concentration of ligands by Faraday's law, it was equal to 2,5 hours. Processes were held in the inert atmosphere in tight system.
After the electrochemical synthesis was finished, the white color slightly soluble complexes which precipitated out, were filtered on the glass filter, were washed with acetonitrile and dried in the vacuum furnace at a temperature 30-50°С.
The terbium (III) content in the obtained complex compounds was calculated by the complexometric titration method.
The carbon and hydrogen content was determined by method of the element microanalysis on C,H,N-analyzer VARIO MICRO CUBE in oxygen current at a temperature of furnace - 1200 °C.
The complex compounds analysis data shows that the complexes content corresponds to the TbL3 general formula.
Complexes and ligands IR-spectra were recorded on IR-Fourier spectrometer VERTEX 70 (Bruker) in 4000-400 cm-1 field. Solid, using attenuated total inside reflection adaptor with diamond crystal.
Ranges of luminescence initiation and registration were recorded at room temperature on a spectrofluorimeter the Flyuorat-02-Panorama (Lumex). Solid samples of obtained complex compounds were used for recording.
Conclusions
1. Using the electrochemical anodic synthesis method there were for the first time obtained waterless of terbium (III) with fluorophenylacetic acids of content TbL3 complex compounds.
2. Using the IR-spectroscopy analysis method the bidentate way for fluorophenylacetic acids with Tb3+ coordination was established.
3. It was found out that terbium (III) with pentafluoroacetic acid complex compound has the highest luminescence.
References
- V.F. Zoliv, L.N. Puntus et al. Z. Alloys and Compounds, 380, 279 (2004).
- Z.–M. Wang, G.R. Choppin. Imarganica Chimica Acta. 293, 167 (1999).
- V.F Zolin, Z. Alloys and Compounds, 380, 101 (2004).
- Katkova M.A., Vitukhnovsky A. G., Bochkarev M. N. // Russ. Chem. Rev. 2005. Vol. 74. N 12. P. 1089. DOI: 10.1070/RC2005v074n12ABEH002481.
- Frolov V.Yu., Oflidi A.I., Bolotin S.N., Shestavin A.I., Panyushkin V.T. // Russ. J. Appl. Chem. 2008. Vol. 81. N 4. P. 639. DOI: 10.1134/S1070427208040137.
- Nakamoto Kazuo // Infrared and Raman spectra of inorganic and coordination compounds, 4th ed., Wiley – New York – 1986 – 484 p.
- Hilder M., Junk P. C., Kynast U.H., Lezhnina M.M.// Journal of Photochemistry and Photobiology A: Chemistry. – 2009. – V.202. – P. 10-20.
Recommended for publication by Prof. S. M. Igumnov
Fluorine Notes, 2019, 122, 5-6
