Received: October 2018
DOI 10.17677/fn20714807.2018.06.03
Fluorine Notes, 2018, 121, 5-6
Laser formation of light guides in electro optical polymers with fluorine containing chromophores in a side chain
V.I. Sokolov 1,2, A.S. Ahmanov1,2, I. M. Asharchuk 1, I.O.Goriachuk1, I.V. Zavarzin3, J. E. Pogodina 3, E.V. Polunin3
1 – Institute of Photon Technologies of Federal Scientific Research Centre «Crystallography and Photonics» of Russian Academy of Sciences, 119333, Leninsky ave. 59, Moscow, Russia
2 – Scientific Research Institute for System Analysis of the Russian Academy of Sciences, 117218, Nakhimovskii prosp., 36/1, Moscow, Russia
3 - N.D. Zelinsky Institute of Organic Chemistry Russian Academy of Science, 119991, Leninsky ave., 47, Moscow, Russia
Abstract: New electro-optical polymers with fluorine containing chromophores in a side chain were synthesized. It was shown that under the influence of visible range laser radiation electro-optical polymers translucence takes place, what happens due to not-reversible chromophores destruction. It was found out that, during the photo destruction process, the polymeric materials refractive index n decreases. Optical waveguides, waveguide splitters, directional couplers, Mach-Zender interferometers and other integrated optical devices elements were formed from electro-optical polymers using the photo-clarification method in light-guiding films.
Keywords: fluorine containing chromophores, electro-optical polymers, photo-bleaching, polymeric waveguides.
Introduction
Electro-optical polymeric materials find a wide application when producing high-speed integrated-optical modulators, transfers, marshalling terminals for telecommunication С – diapason of wavelength 1530 – 1565 nm [1 - 8]. Such materials are produced with the usage of chromophores, which are able to change refractive index under the influence of applied electric field. Electro-optical polymer can be produced either by inputting chromophores molecules into the passive matrix (guest – host system) [4 - 6], or by its covalent integration into the polymeric macromolecule side chains (side – chain system) [7, 9]. The second approach is more perspective, because it prevents chromophores agglomeration which decreases electric coefficient r33. The synthesis of new electro-optical chromophores, which along with the high electro optical coefficient r33, have high optical transparency in C-diapason, is significant. We suggest to use fluorine containing chromophores in order to increase optical transparency. The idea of such approach is that the substitution of more lightweight hydrogen atoms on more heavy fluorine atoms leads to oscillative overtones dislocation to the side of longer wavelength [10]. As a result, electro-optical polymeric material’s transmission windows open in C – spectrum field. Besides that, the hydrogen atoms for fluorine atoms substitution raises chromophores molecule acceptor part electronegativity at the same time decreasing its dipole moment.
The present article reports about the synthesis of new chromophores – «disperse red» DR-3F (Disperse Red 3F) and disperse orange DO-2 (Disperse Orange 2) and also about the electro-optical polymeric materials of «side-chain» type, produced on the base of these chromophores. These materials are represented as polymethylacrylate with covalently integrated chromophores DO-2 and DR-3F in a side chain. Light-guiding films with the thickness 0.5 – 4 μm were prepared from electro-optical polymers. It was shown that under the laser radiation with the wavelength 440 nm influence, electro-optical polymers clarification happens. Such clarification is explained by not-reversible chromophores DR-3F and DO-2 photo destruction. It was found out that during the photo destruction process, the refractive index n of polymeric material decreases and the n variation may achieve n = 0.011. This allows to prepare channel optical waveguides and other integrated optical devices elements using the selective laser photo clarification.
1. Synthesis of PMMA with covalently integrated fluorine containing chromophores
The synthesis of PMMA with fluorine containing electrooptical chromophores DR-3F and in DO-2 in a side chain was held in three stages. Chromophore was synthesized at the first stage. At the second stage, the given chromophore was covalently integrated to methacryloylchloride molecule. At the third stage, the radical thermal co-polymerization of obtained ether and methylmethacrylate with linear electrooptical polymer formation was held. The described stages of PMMA/DR-3F polymer synthesis are shown in the Pic. 1.
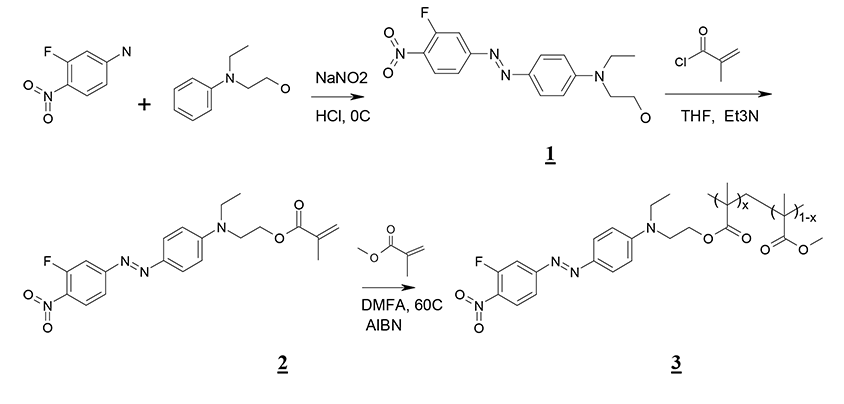
Pic. 1. Scheme of PММА polymer with fluorine containing chromophore DR-3F in a side chain synthesis. Compound 1 – chromophore DR-3F, 2 – methacrylic ether with covalently integrated chromophore DR-3F, 3 – polymer PMMA/ DR-3F, x – elements molar concentration with DR-3F in PMMA/DR-3F polymer macromolecule.
3-Fluoro-4-nitro-4’(N-ethyl-N-oxyethylamino)azobenzene (1)
4-Nitro-3-fluoroaniline (2,3 g) were dissolved in 7,67 ml of hydrochloric acid and 4,5 ml of water mixture. Cooled to 0°С. Then, 1,04 g of sodium nitrite in 10 ml of water were being added drop by drop during 20 min. The solution was being mixed during 30 min at 0°С. N-ethyl-N-hydroxyethylaniline was dissolved in 2,4 ml of 10% hydrochloric acid and during 1,5 hour was being added to diazonium salt solution and mixed during 1 hour. Then sodium hydroxide 10% solution was being added until precipitate formation. The precipitate was filtered, washed in 2х20 ml of water and dried on air until the constant mass. Obtained 4,31 g of compound 1 as dark-red crystals, melting temp. 135-137°С, yield 86,4%.
1Н NMR spectra: (DMSO–d6): 1,22(t, 3H), 3,5-3.7(m, 6H), 4,9(t, 1H), 6,9(d, 2H), 7,75(m, 2H), 7.85(d, 2H), 8,3(t, 1H).
4-(3-Fluoro-4-nitrophenylazo)-N-ethyl-N-(2-methaacrylyloxyethyl)aniline (2)
Compound 1 (2,84 g) was dissolved in 5 ml of THF, 1,3 ml of triethylamine were added. Cooled to 0°С. Fresh-prepared methacryloylchloride (1 ml) solution was being added drop by drop into 3 ml of THF and was being mixed during 24 hours at a room temperature. The precipitate was filtered, washed in THF and vaporized in vacuum till the constant mass. The rest was dissolved in CHCl3, the solution was washed with water (3х20 ml) and was vaporized till the constant mass at 40°С. Obtained 1,54 g of product 2 as dark-red crystals, melting temp 73-75°С. Yield 57%.
1Н NMR spectra: (CDCl3): 1.28(t, 3H), 3.55(q, 2H), 3.75(t, 2H), 4.4(t, 2H), 5.6(s, 1H), 6.13(s, 1H), 6.8 (d, 2H), 7,73(d, 2H), 7.95 (m, 4H).
Ether’s copolymer 2 with methyl methacrylate (3)
Compound 2 (0,75 g, 1.85 mM) was dissolved in 4 ml of DMF, there were added 1,77 ml (16,7 mM) of methyl methacrylate and 75 mg. of AIBN. After mixing at 60°C during 24 hours the reaction mixture was put in cooled up to 0°С methanol, the precipitate was filtered, washed with methanol and dried on air to the constant mass. 2,05 g. of the product 3 (х = 0.08) were obtained as dark-red powder. Yield 85%.
1Н NMR spectra: (CDCl3): 1.28(t, 3H), 1,75 (м, СН3 chain), 7Н(2СН2N+3CH3O chain), 4,2(м, 2H), 6.8(d, 2H), ), 7,73(d, 2H), 7.95(m, 4H).
In the Pic. 2 the stages of PMMA/DO-2 electro-optical polymer synthesis are shown.
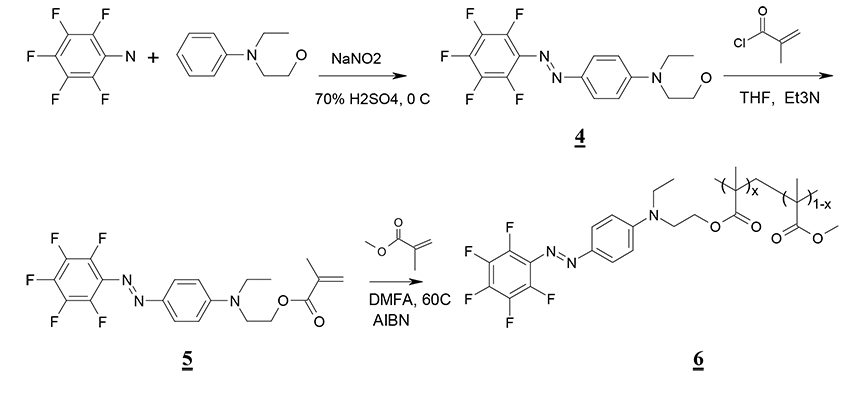
Pic. 2. Scheme of the PММА polymer with fluorine containing chromophore DO-2 in a side chain synthesis. 4 – chromophore DO-2, 5 – methacrylic ether with covalently integrated chromophore DO-2, 6 – polymer PMMA/DO-2, x – elements molar concentration with DO-2 in PMMA/DO-2 polymer macromolecule.
4-Pentafluorophenylazo-N-ethyl-N-(2-hydroxyethyl)aniline (4)
Pentafluoroaniline (5 g) was dissolved in 70% H2SO4 (10,3 ml). Cooled in ice bath to 0°С. During 2 hours NaNO2 (2,26 g) was being added in small portions. 4,31 ml of N-ethyl-N-hydroxyethylaniline were dissolved in 70% of sulfuric acid (8,3 ml) and during 20 min this solution was being added to the reaction mixture at 4°С. The reaction mixture was being mixed during 50 min. 50 ml of water and NaHCO3 were being added till the reaction mixture рН 8. Extracted CHCl3 3х40 ml, dried over Na2SO4 during 24 hours, after this, separated on the chromatographic column with SiO2 (40-100 μm, D 30 mm, H 400 mm), eluent – ethyl acetate-petroleum ether 1:1. Containing product fractions Rf 0.27 (SiO2, ethyl acetate-petroleum ether 1:1) combined and vaporized in vacuum. Obtained 0,84 g of compound 4 as dark-brown viscous oil, which, according to NMR data, contains, 40% of initial N-ethyl-N-hydroxyethylaniline. Yield 8,6%.
1Н NMR spectra 4: (CDCl3): 1.25(t, 3H), 3.5-3.7(m, 4H), 3.90(t, 2H), 6.65(d, 2H), 8.1(d, 2H). 19F NMR spectra: (CDCl3): -152.7(2F), -157.5(1F), -163.9(2F).
4-Pentafluorophenylazo-N-ethyl-N-(2-methacryloxyethyl)aniline (5)
Compound 4 (0,84 g) was dissolved in 5 ml of THF, there were added 0,9 ml of triethylamine. Cooled to 0°С. 0,68 ml of methacryloylchloride in 3 ml of THF freshly-prepared solution was being added drop by drop and then the solution was being mixed during 24 hours at a room temperature. The precipitant was filtered, washed in THF and vaporized in vacuum till the constant mass. The rest was dissolved in CHCl3, the solution was washed with water (3х20 ml) and vaporized in vacuum till the constant mass at 40°С. Obtained 0,99 g of product 5 as dark-red oil, Rf 0,60 (SiO2, ethyl acetate-petroleum ether 1:2). Yield 95%.
1Н NMR spectra 5: (CDCl3): 1.25(t, 3H), 1.93(s, CH3), 3.5-3.7(m, 4H), 3.90(t, 2H), 5.68(s, 1H), 6.18(s, 1H), 6.65(d, 2H), 8.1(d, 2H).
Ether’s copolymer 5 with methyl methacrylate (3)
Compound 5 (0,95 g, 2,34 mM) was dissolved in 4 ml of DMF, there were added 2,23 ml (21,1 mM) of methyl methacrylate and 75 mg. of AIBN. After mixing at 60°C during 24 hours the reaction mixture was put in cooled up to 0°С methanol, the precipitate was filtered, washed with methanol and dried on air to the constant mass. 1,19 g. of the product 6 were obtained as orange powder. Yield 54%. The product contains 2 mol% chromophore (х = 0,02), what was found out by correlation of integral intensities of chromophore fragment aromatic protons signals and protons of OCH3-group of polymer methacrylic chain links.
In order to count the overage molecular mass Mw of synthesized electro-optical polymers the PMMA/DR-3F and PMMA/DO-2, hydrodynamic diameters D were measured in dichloromethane (globules diameters), using the 90Plus_Zeta (Brookhaven Instruments Corp., USA) analyzer. Dichloromethane was chosen as a solvent because PMMA is easily dissolved in it, at the same time the dichloromethane refractive index nD = 1.4244 at 20 0С varies from the polymers PMMA, PMMA/DR-3F and PMMA/DO-2 refractive index. In the Pic. 3 macromolecules distribution of the given polymers in dependence to D is shown. By way of comparison, in this picture the macromolecules distribution for polymer PMMA brand ACRYREX CM-205 is given. As it is shown in the picture 3, the Z-average diameter of the molecules PMMA/DO2 is equal to Davr = 5.1 Nm, PMMA/DR-3F - 6.4 Nm, when for ACRYREX CM-205 Davr = 15 Nm. Taking into account that for the last Mw 5 × 105 g/mole [10], molecular mass of the polymer PMMA/ DR-3F can be calculated as Mw 1.2 × 105 g/mole, PMMA/DO2 as Mw 6 × 104 g/mole.

Рic. 3. The allocation G(D) of macromolecules of the synthesized polymers PMMA/DO-2 (1) and PММА/DR-3F (2) depending on its D globules sizes in dichloromethane, measured using the dynamic light scattering method. The corresponding allocation for polymer PMMA brand ACRYREX CM-205 (3) с Mw 5 × 105 g/mole is given for comparison.
Diffraction spectrum of 3 and 6 electro-optical polymers were measured on X-ray diffractometer Rigaku miniflex 600, shown in the Pic. 4. One can see that PММА/DR-3F spectra does not have sharp diffraction peaks, at the same time one can observe broad halo with centers near 2 17, 22 and 43 deg. Besides halo, located near 13, 30 and 43 deg, in PMMA/DO-2 spectra one can also observe sharp peaks (at 2 27.8, 29.3, 31.7 deg and etc.). According to the given diffractograms, we can conclude that PММА/DR-3F polymer is amorphous, whether PMMA/DO-2 polymer is a mixture of amorphous and polycrystalline phases.
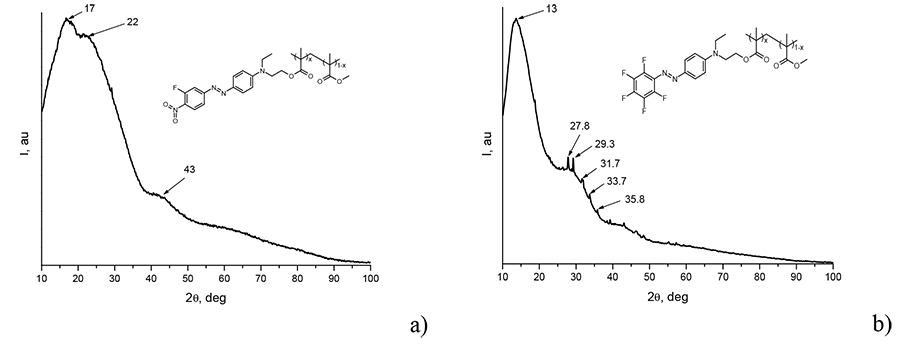
Pic. 4. PММА/DR-3F with x = 0.08 (а) and PMMA/DO-2 with x = 0.02 (б) polymers diffractograms, recorded on Rigaku Miniflex600 ( = 1.54178 A).
2. Electro-optical polymers with covalently integrated fluorine containing chromophores laser photo clarification
Using the centrifuge method, the light-guiding films were formed from the synthesized electrooptical
polymer with thickness Hf from 0.5 to 4 μm from solutions of PMMA/DR-3F and PMMA/DO-2
in chlorobenzene on quartz substrates, and also on silicon substrates with thermal grown oxide layer.
Absorption spectra of freshly applied on the substrate SiO2 film PMMA/DR-3F and PMMA/DO-2
are illustrated by the curve 1 in the Рic. 5a and Pic. 5b correspondingly. We can see that electrooptical
polymers have intensive absorption bands with the centers near 482 nm and 287 nm (PMMA/DR-3F), 427
and 262 nm (PMMA/DO-2), which are due to the light absorption by chromophore molecules DR-3F and
DO-2.
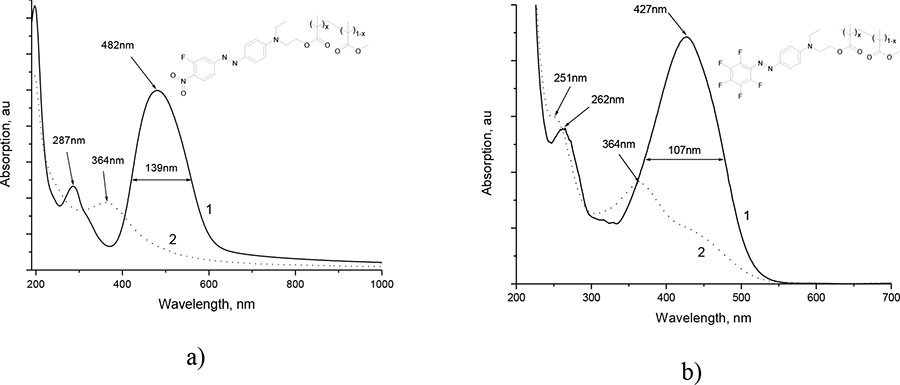
Pic. 5. (а) Absorption spectra of light-guiding film PММА/DR-3F from x = 0.08 to (1) and after (2) exposing with laser radiation with wavelength 440 nm. (b) The same for PMMA/DO-2 with x = 0.02. In the insertions there are shown the molecular structures of the electrooptical polymers.
In the Pic. 5 there are shown the absorption spectra of the given films after exposing by diode laser radiation with the wavelength 440 nm (curves 2). From curves 1 and 2 comparison in the Pic. 5a, it follows, that during the exposing process, the absorption band intensity with the centers near 482 and 487 nm decreases, however new absorption band near 364 appears. Such spectrum absorption variation, with material discoloration, is probably due to inconvertible chromophore DR-3F photodegradation with the loss of color (photo bleaching effect [6, 11]). Polymer PMMA/DO-2 suffers similar changes during the photo clarification process (Pic. 5b)
The polymers, containing DR-3F and DO-2 chromophores, photo clarification is followed by its refractivity n variation. This variation depends on molar concentration of x chromophore in macromolecule and may achieve n = 0.011 (for PММА/DR-3F with x = 0.08).
Such refractive index variation is enough for the formation of channel optical waveguides with numerical aperture NA = 0.16 and higher and of other integrated optical devices elements (waveguide splitters, directional couplers and so on). The process of the formation is as follows: under the influence of space-selective photo clarification in the film there are formed fields with the lower refractive index, used as waveguide’s cover, weather unexposed electrooptical material is a light-guiding core, in which optical radiation spreads. The channel optical waveguides array, formed in the film from PMMA/DR-3F under the influence of laser radiation with the wavelength 440 nm is shown in the Pic. 6.
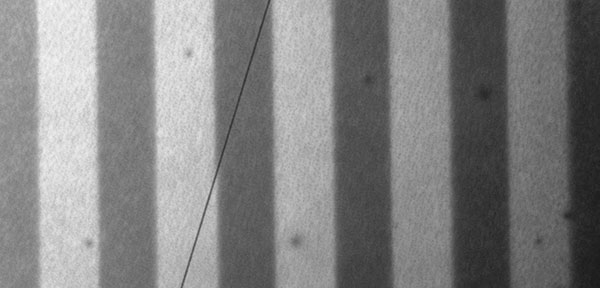
Pic. 6. The channel optical waveguides array, formed in the film from PMMA/DR-3F, under the influence of laser radiation with the wavelength 440 nm. Dark bands with the thickness 100 μm (unclarified parts) are waveguides light-guiding cores.
Consequently, by using the laser photoclarification of electrooptical polymer films PMMA/DR-3F and PMMA/DO-2 there can be produced different elements of integral-optical devices, among them Mach-Zender waveguides interferometers.
Conclusion
The new fluorine containing electro optical chromophores DR-3F and DO-2 were synthesized. The methodology for obtainment of electrooptical polymers on the base of PMMA with covalently integrated chromophores DR-3F and DO-2 in the side chain was developed. Light-guiding films were prepared from these polymers. It was shown, that under the influence of the laser radiation with the wavelength 440 nm, photo bleaching of polymers happens, what allows to form various waveguides integrated optical devises elements under the laser radiation influence.
Acknowledgements
This work was supported by the Ministry of Science and Higher Education within the State assignment FSRC «Crystallography and Photonics» RAS in part of investigation of properties of electro-optical chromophores, RFBR (grant 17-07-01478) in part of chromophores synthesis, RFBR (grant 18-32-00948) in part of laser writing in electro-optical polymer materials.
References
- Dalton. L., Benight S. Theory-Guided Design of Organic Electro-Optic Materials and Devices // Polymers. 2011. V. 3. P. 1325. 10.3390/polym3031325.
- Liu J., Xu G., Liu F., Kityk I., Liu X., Zhen Z. Recent advances in polymer electro-optic modulators // RSC Advances. 2015. V. 5, P. 1578. 10.1039/C4RA13250E.
- Zhang H., Oh M.C., Szep A., Steier W.H., Zhang C., Dalton L.R., Erlig H., Chang Y., Chang D.H., Fetterman H.R. Push-pull electro-optic polymer modulators with low half-wave voltage and low loss at both 1310 and 1550 nm // Applied Physics Letters. 2001. V. 78. № 20. P. 3136. 10.1063/1.1372203.
- Zheng C.T., Zhang L.J., Qv L.C., Liang L., Ma C.S., Zhang D.M., Cui Z.C. Nanosecond polymer Mach-Zehnder interferometer electro-optic modulator using optimized micro-strip line electrode // Opt. Quant. Electron. 2013. V. 45. № 3. P. 279. 10.1007/s11082-012-9629-1.
- Nazmieva G.N., Vakhonina T.A., Ivanova N.V., Mukhtarov A.Sh., Smirnov N.N., Yakimansky A.V., Balakina M.Yu., Sinyashin O.G. Testing of the ways for synthesis of new nonlinear optical epoxy-based polymers with azochromophores in the side chain // European Polymer Journal. 2015. V. 63. P. 207-216.
- Sokolov V.I., Akhmanov A.S., Asharchuk I.M., Goryachuk I.O., Khaydukov K.V., Nazarov M.M. Formation of channel optical waveguides in polymethylmethacrylate with embedded electro-optic chromophore DR13 by the photoinduced bleaching method // Optics and Spectroscopy. 2017. V. 122. № 3. P. 469–474.
- Michel S., Zyss J., Ledoux-Rak I., Nguyen C.T. High-performance electro-optic modulators realized with a commercial side-chain DR1-PMMA electro-optic copolymer // Proceedings of SPIE. Organic Photonic Materials and Devices XII. 2010. V. 7599. P. 75990I. 10.1117/12.841339.
- Denisyuk I.Y., Burunkova Y.E., Pozdnyakova S.A., Balya V.K., Zhuk D.I., Fokina M.I. An electro-optic polymer modulator for radio photonics // Optics and spectroscopy. 2015. V. 119. № 4. P. 719-723. 10.7868/S0030403415100104.
- V.I. Sokolov, A.S. Ahmanov, Е.S. Vasilenko, I.O.Goriachuk, S.I. Molchanova, J. E. Pogodina, E.V. Polunin. Synthesis and investigation of optical properties of fluorine containing chromophore Disperse Orange DO1 // Fluorine Notes. 2018. V 5(120). P. 1. 10.17677/fn20714807.2018.05.01
- W. Groh. Overtone absorption in macromolecules for polymer optical fibers // Makromol. Chem. 1988. V. 189. P. 2861 – 2874. 10.1002/macp.1988.021891213.
- J. Liu, G. Xu, F. Liu, I. Kityk, X. Liu, Z. Zhen. Recent advances in polymer electro-optic modulators // RSC Advances. 2015. V. 5. P. 15784-15794. 10.1039/C4RA13250E.
- Nakanishi M., Sugihara O., Okamoto N., Hirota K. Ultraviolet photobleaching process of azo dyedoped polymer and silica films for fabrication of nonlinear optical waveguides // Applied Optics. 1998. V. 37. № 6, P. 1068. 10.1364/AO.37.001068.
- K.S. Levchenko, K.A. Chudov, E.V. Zinoviev, K.A. Lyssenko, D.U.Demin, N.O. Poroshin, P. Grebennikov. Synthesis of unsymmetrical 4-oxo-2-vinyl-4H-chromene-3-carbonitrile dyes via Knoevenagel reaction // Tetrahedron Letters. 2018. V. 59, p. 2788-2792. 10.1016/j.tetlet.2018.06.012.
Recommended for publication by Prof. S.M. Igumnov
Fluorine Notes, 2018, 121, 5-6
