Received: July 2018
DOI 10.17677/fn20714807.2018.04.03
Fluorine Notes, 2018, 119, 5-6
Modification of the amino group in polyfluorinated arylenediamines by reactions with aldehydes and quinones
T.A. Vaganova, I.P. Chuikov, E.V. Malykhin*
N. N. Vorozhtsov Novosibirsk Institute of Organic Chemistry, Siberian Branch of the Russian Academy of Sciences, Lavrentiev Avenue 9, 630090 Novosibirsk, Russian Federation
E-mail: malykhin@nioch.nsc.ru
Abstract: Reaction of low-reactive polyfluoroaromatic diamines with benzaldehyde and hexafluoronaphthoquinone provide selective preparation of functionalized arylamines. Mono-N-benzylidene-arylenediamines were prepared in 30-35 % yield, polyfluoroarylaminonaphthoquinones in 50-55 % yield. The products were characterized by IR, 1H and 19F NMR spectroscopy, mass-spectrometry. Spectral characteristics of the compounds in the UV-visible range were studied and prospects of their usage as signal components of supramolecular sensors were evaluated.
Keywords: Polufluorinated arylenediamines, mono-N-benzylidene-polyfluoroarylenediamines, polyfluoroarylaminonaphthoquinones, chromophores.
Polyhalogenaromatic mono- and diamines are widely used as structural blocks in the synthesis of high-tech materials, pharmaceuticals and agrochemicals [1]. Organic co-crystals seems to be promising objects for high technologies, materials possessing NLO, photochromic, magnetic and other useful properties are developed on their basis [2]. For example, the effect of reversible rearrangement of the supramolecular structure (solid-state transition) under the action of an external force can be used in thermo- and photochromic molecular sensors and switches [3]. We proposed and studied in detail a group of new objects for crystal engineering – associates of polyhalogenaromatic mono- and diamines with 18-crown-6, which are supramolecular hydrogen-bonded assembles [4,5]. For a number of substituted polyfluoroarylamines, significant changes in the fluorescence characteristics, both in intensity and wavelength, were observed in going from the free amine to the associate with 18-crown-6 [5]. These effects open the possibility to use the supramolecular assembles in developing solid-phase chemo-specific supramolecular indicators and sensors, where two simple organic compounds connected by the hydrogen bond performe their receptor and signal functions. Destruction of the intermolecular bond due to the capture of an analyte by the macrocycle receptor is accompanied by a change in the detectable characteristics of the signal group. To increase the sensitivity of the sensor, it is important to search for signal amine components that have effective chromo- or fluorophore properties.
A rational approach to the synthesis of functionalized polyhalogenarylamines is the modification of one of the two amino groups in arylenediamines. Polyhalogenated arylenediamines are available compounds as there is a convenient and efficient method for their synthesis – mono- and bis-aminodefluorination of polyhalogenarenes of the benzene, naphthalene and pyridine series with anhydrous ammonia used as a reagent and a solvent in the same time [6-8]. The most obvious ways of functionalizing of the arylenediamines at the amino group to form chromo- and fluorophore systems, including conjugated ones, are their reactions with aldehydes and quinones. Condensation of amines with aldehydes to form aldimines (N-arylideneanilines) has a large potential due to the possibility of a wide variation in the structure of aromatic substituents at the polar double bond C=N, which gives a tool to control their properties. Aromatic aldimines, including those containing polyfluorinated fragments, are known as objects for supramolecular systems, liquid crystal media, molecular devices, etc. [9]. Effective quinonoid type chromo- and fluorophores can be attached to the amino group by nucleophilic substitution of a hydrogen or a halogen atom [10-14]. In addition to the stated objective, the combination of the amino group, polyfluoroarene and quinone fragments in the molecule is of interest for the study of its bioactivity [10-12]. The goal of this work is to study a possibility of the synthesis of functionalized polyfluoroarylamines by reactions of arylenediamines with aldehydes and quinones.
Results and discussion
A priori there are two problems that get in the way of the preparative use of the above reactions: low reactivity of the amino group in polyfluorinated substrates and the necessity to limit a conversion to a modification of only one of the two amino groups. It is known that the exhaustive fluorination of the benzene ring reduces the reactivity of phenylenediamines to acylating agents by a factor of 105 as a result of the total acceptor effect of electronegative fluorine atoms [15]. For this reason pentafluoroaniline does not react with benzaldehyde under conditions typical for non-fluorinated arylamines. To synthesize imines, pentafluoroaniline is preliminary treated with thionyl chloride to form N-sulfinylpentafluoroaniline, which then gives the desired products in high yields by the reaction with aromatic aldehydes [16]. However, the applicability of this approach for the modification of one of the two amino groups in arylenediamines is not obvious. In principle, the reaction of pentafluoroaniline with aldehydes can be carried out by prolonged refluxing in anhydrous solvents with water-removing agents (in the presence of MgSO4 in CH2Cl2 [17], molecular sieves in toluene [18]), or by heating at 160 °C without solvent as well. [19]. Preparation of N,N'-bis-benzylidene derivatives of tetrafluoro-meta-phenylenediamine was also reported [20]. Examples of the arylenediamine modification when only one of the amino groups remains unchanged have not been described in literature.
To study the reactions with benzaldehyde as a model reagent, polyfluoroarylenediamines with three basic types of the aromatic framework were selected (Scheme 1): 2,5-difluoro-1,3-phenylenediamine (1a), 2,6-diamino-3,5-difluoropyridine (2a), and 2,7-diaminohexafluoronaphthalene (3a). Anhydrous toluene was used as a solvent and three types of water-removing agents, i.e. P2O5, MgSO4, and activated molecular sieves. The conversion was monitored by 19F NMR spectroscopy.
Activity of polyfluorinated substrates against benzaldehyde, as expected, was low: after heating at 110 ° C for 24 hours in the presence of molecular sieves, from 50 % (1a) to 70 % (2a and 3a) of the unreacted diamine remain in the reaction mass. P2O5 and MgS04 accelerate the condensation but cause appreciable resinification of the reaction mixture. The most effective and gentle way for removing water is azeotropic distillation with the solvent; these conditions allow the full conversion of benzaldehyde to be achieved in ~10 hours. In all the cases, as mono-N-benzylidene-arylenediamine (compounds 1b, 2b, 3b) accumulates in the reaction mixture, the free amino group in the product undergoes a modification in parallel with the amino group in the starting diamine. With an increase of the reaction time, the ratio of bis- to mono-modified products grows, regardless of the way of the water removing. Varying the temperature in the range of 80-110 ° C also has no significant effect on the reaction selectivity.
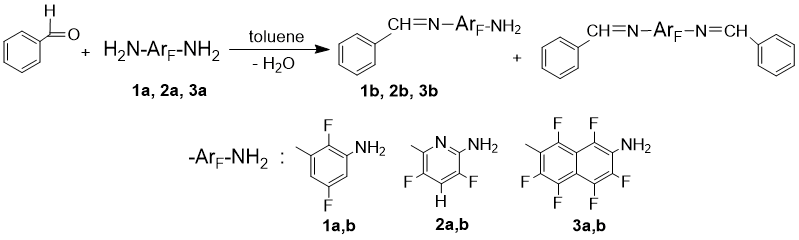
Scheme 1. Reaction of polyfluoroarylenediamines with benzaldehyde.
Based on the optimization results, the following mode was selected for the synthesis of mono-N-benzylidene-arylenediamines: use of 20% excess of the substrate relative to the aldehyde, removing the water formed during the condensation as an azeotrope with toluene (solvent), termination of the reaction at 60-70% conversion. After this, unreacted benzaldehyde was distilled off with toluene under reduced pressure, and a mixture of mono- and bis-imines with the starting diamine was obtained (the approximate molar ratio is 5: 1: 5 respectively, according to 19F NMR and GC-MS). Due to the significant difference in polarity of these compounds, the target mono-N-benzylidene-arylenediamine can be isolated by chromatography using a mixture of anhydrous eluents hexane + diethyl ether with a polarity gradient 12÷5 : 1 respectively. The first fractions of eluate contain a trace amount of benzaldehyde and bis-imine, intermediate – a mixture of bis- and monoimines, then a practically pure monoimine is isolated, the most polar diamine is eluated with diethyl ether. In this manner, mono-N-benzylidene-arylenediamines 1b, 2b, 3b (Scheme 1) were obtained in a yield of ~30% (see Experimental). The compounds were synthesized for the first time and characterized with a set of spectral methods. It should be noted that the imines obtained are sensitive to air moisture and residual moisture in solvents. When studying the optical characteristics of the substances in the UV-visible range (solutions in acetonitrile, c = 10-4 M), low-intensity absorption bands of the corresponding diamines were recorded in the spectra, their intensity being increased upon solution standing. This is due, most likely, to hydrolysis of the imine function. For this reason, the spectral characteristics of mono-N-benzylidene-arylenediamines 1b, 2b, 3b were obtained by subtracting the spectra of the starting diamines from the spectra of the experimental samples. The exact ratio of the compounds in solutions could not be determined in all cases, therefore the optical densities are given as an intensity indicator for the absorption bands in the UV spectra of imines 1b and 3b. It follows from the data obtained that mono-N-benzylidene-arylenediamines 1b, 2b, 3b have absorption bands in the longer wavelength region than the starting diamines (the difference between λmax is 40-80 nm, spectral characteristics are presented in Experimental), which is the consequence of increasing the length of the conjugated system. Thus, it was shown by the model reaction, that the proposed method of modification of polyfluorinated arylenediamines can be used to synthesize the amine-containing chromophores in the visible range.
Reaction of non-fluorinated arylamines with benzoquinone occurs in methanol at room temperature and result in the formation of 2,5-bis-amino derivatives in high yields [10]. Substitution of halogens in polyhalogenated (Hal = Cl, F) benzo- and naphthoquinones for various uncharged nucleophiles – amines, alcohols, thioalcohols – proceeds even faster [11, 13]. We used polyfluorinated arylenediamines with different framework and the number of fluorine atoms as nucleophiles: 2,5-difluoro-1,3-phenylenediamine (1a), 2,7-diaminohexafluoronaphthalene (3a), 2,4,5-trifluoro-1,3-phenylenediamine (4a), 4-chloro-trifluoro-1,3-phenylenediamine (5a), and 2,4-diaminotrifluoropyridine (6a). It was found that para-benzoquinone does not react with these diamines even under refluxing in toluene for 72 hours; the starting compounds were returned. Hexafluoro-1,4-naphthoquinone (7) also gives no substitution products under the action of the exhaustively halogenated diamines 3a, 5a, 6a in tetrahydrofuran under heating. The addition of triethylamine to bind HF does not affect the result. Within a day, naphthoquinone 7 is completely destroyed under these conditions while diamines are unchanged. In contrast, the more nucleophilic compounds 1a and 4a with one of the ortho-positions to the amino group not occupied by a fluorine atom, react with naphthoquinone 7 in the presence of NEt3 in tetrahydrofuran at 45-50 °C in an argon atmosphere to form polyfluoroarylaminonaphthoquinones 1c and 4c (Scheme 2). The highest yield of the products is observed when a reaction time is 20 h. It should be noted that this conversion, in contrast to the reaction with benzaldehyde, occurs selectively at one amino group; polyfluorinated bis-quinone derivatives of arylenediamines are not detected. The isomer selectivity in the reaction of non-symmetric phenylenediamine 4a is also high, only the amino group having a hydrogen atom at the ortho-position undergoes modification. Compounds 1c and 4c, synthesized for the first time, were isolated by chromatography in yields of 50-60% and were characterized by spectral methods.
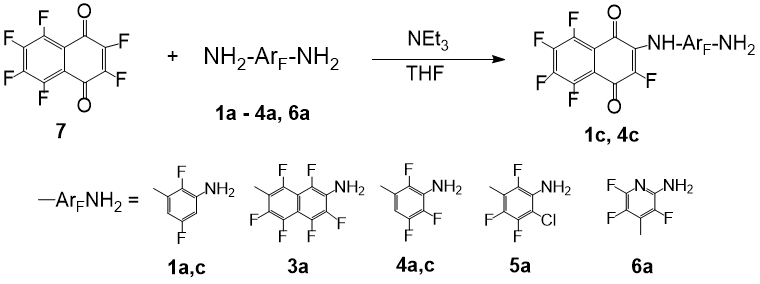
Scheme 2. Reaction of polyfluoroarylenediamines with hexafluoronaphthoquinone.
Compounds 1c and 4c comprising a pentafluoronaphthoquinone fragment have a burgundy color, the color of their concentrated tetrahydrofuran solutions turns into dark orange when diluted by ~3 orders of magnitude. The absorption spectra of these compounds in the UV-visible range differ from those of diamines 1a, 4a, and quinone 7. In the spectra of polyfluoraminonaphthoquinones 1c and 4c, the new absorption maximum at 455-460 nm appears, which is located in the longer wavelength region in comparison to the characteristics of the starting compounds (the difference between λmax is 110 nm). This indicates the interaction of a lone electron pair on the nitrogen atom with π-systems of the quinone and benzene fragments. Thus, the reaction of partially fluorinated arylenediamines with naphthoquinone 7 is an acceptable method for the synthesis of bipolar chromophores with a long conjugation chain.
The results obtained show that the reactions with aldehydes and polyfluoroquinones can be used to modify one of the amino groups in the low reactive polyfluoroarylenediamines to form chromo- and fluorophore systems. The yields of the target products in the reactions with benzaldehyde are 30-35%, and in the reactions of hexafluoronaphthoquinone with polyfluorophenylenediamines that contain no fluorine atom at the ortho-position to the amino group – 50-55%. Polyfluorinated arylaminonaphthoquinones such as 1c and 4c appear to be promising signal components for supramolecular sensors, since lone electron pairs on the nitrogen atoms of amino groups ensure the formation of a bipolar chromophore with an extended π-system and, accordingly, the transfer of electronic effects when forming/breaking the associates with a macrocyclic receptor. The possibility of using mono-N-benzylidene-arylenediamines 1b, 2b, and 3b to make supramolecular objects is limited by their high sensitivity to moisture. Nevertheless, this method is in principle productive for the preparation of conjugated chromo- and fluorophore polyfluoroarylamines. Development of this synthetic approach requires searching for such pairs of reagents (diamines and aldehydes) which will ensure the formation of relatively stable aldimines.
Experimental
1H and 19F NMR spectra were recorded on a NMR spectrometer Bruker AV-300 (300.13 and 282.36 MHz, respectively) using residual proton signals of the deuterated solvent relative to TMS (δ = 0 ppm) and C6F6 (δ = 0 ppm) as internal standard. Fourier transform infrared (FTIR) spectra were measured on a Bruker Tensor 27 instrument for KBr pellets. UV-vis spectra were recorded on a Varian Cary 5000 spectrometer for solutions of samples in acetonitrile. The precise molecular weights of ions were determined by high resolution mass spectrometry on a Thermo Scientific DFS instrument, EI, 70 eV. GC-MS analysis was performed using a Hewlett Packard G1081A equipment comprising an HP 5890 Series II gas chromatograph and an HP5971 mass selective detector; electron ionization energy of 70 eV; HP5 column (5% of biphenyl and 95% of dimethylsiloxane), 30 m×0.25 mm×0.25 μm; with helium as carrier gas, flow rate 1 mL min-1; column temperature programming from 50 ºС (2 min) at an increment of 10 ºС min-1 to 280 ºС (5 min); injector temperature 280 ºС; ion source temperature 173 ºС; data acquisition rate 1.2 scan s-1 in the mass range 30 to 650 amu.
Toluene was dried by boiling over CaCl2 followed by distillation. Tetrahydrofuran was purified by boiling over benzophenone ketyl sodium salt followed by distillation in argon atmosphere. Commercial benzaldehyde and triethylamine were purified using distillation. HPLC grade acetonitrile was dried over CaH2 and distilled before UV spectra recording. 2,6-Diamino-3,5-difluoropyridine (2a), 2,4,5-trifluoro-1,3-phenylenediamine (4a), and 2,4-diamino-3,5,6-trifluoropyridine (6a) were prepared as described in [6], 2,7-diaminohexafluoronaphthalene (3a) – as described in [7], 2,5-difluoro-1,3-phenylenediamine (1a) and 2,4,5-trifluoro-6-chloro-1,3-phenylenediamine (5a) – as described in [8], hexafluoro-1,4-naphthoquinone (7) – as described in [21]; melting points and NMR spectra correspond to the literature data. UV-visible spectra, λmax/nm (logε): 1a 245 (4.0), 279 (3.0); 2a 227 (3.8), 319 (3.9); 3a 219 (4.2), 252 (4.9), 290 (3.6); 4a 205 (4.3), 230 (3.7), 283 (2.8). UV-visible spectra of compound 7 in chloroform, λmax/nm (logε): 246 (3.9), 260 (4.2), 268 (4.2), 343 (3.3) [22].
Reaction of polyfluorinated arylenediamines with benzaldehyde (a typical procedure).
Polyfluorinated arylenediamine (2.4 mmol) was dissolved in toluene (8-10 ml), and benzaldehyde (0.21 g, 2 mmol) was added with stirring. The mixture was stirred at 120 °С for 4-6 h, water being distilled off as azeotrope with solvent. The reaction was terminated after reaching the conversion of arylenediamine 55-60% (according to 19F NMR). Toluene and unreacted benzaldehyde were distilled off under reduced pressure. The product was isolated by column chromatography on SiO2 using a mixture of anhydrous eluents of variable polarity (hexane–diethyl ether = 12÷5 : 1).
N1-benzylidene-2,5-difluoro-1,3-phenylenediamine (1b) was synthesized from diamine 1a (0.35 g), yield 0.15 g (32%). The yellow oil. UV-vis, λmax/nm (D): 261 (0.47), 315 (0.21). 1Н NMR, chloroform-d, δ/ppm, J/Hz: 5.06 (br.s, 2Н, NH2), 6.28 (ddd, 1Н, Н4, JFH = 10, JFH = 6, JHH = 3), 6.52 (ddd, 1Н, Н6, JFH = 9, JFH = 6, JHH = 3), 7.48-7.57 (m, 3H, H3’, H5’, H4’), 8.0 (m, 2H, H2’, H6’), 8.57 (br.s, 1H, C=H). 19F NMR, chloroform-d, δ/ppm, J/Hz: 8.5 (ddd, 1F, F2, JFF = 13, JFH = 6, JFH = 6), 44.5 (ddd, 1F, F5, JFF = 13, JFH = 10, JFH = 9). Found: m/z 232.0816 [M]+. C13H10N2F2. Calculated: M = 232.0812.
N2-benzylidene-3,5-difluoro-2,6-diaminipyridine (2b) was synthesized from diamine 2a (0.35 g), yield 0.17 g (37%). Viscous orange mass, vitrifies when standing. UV-vis, λmax/nm (logε): 268 (4.1), 358 (3.9). 1Н NMR, chloroform-d, δ/ppm, J/Hz: 4.49 (br.s, 2Н, NH2), 7.21 (dd, 1Н, Н4, JFH ~9), 7.45-7.51 (m, 3H, H3’, H5’, H4’), 7.97 (m, 2H, H2’, H6’), 9.08 (br.s, 1H, C=H). 19F NMR, chloroform-d, δ/ppm, J/Hz: 22.5, 24.9 (both d, in 1F, F3 and F5, JFH ~9). Found: m/z 233.0771 [M]+. C12H9N3F2. Calculated: M = 233.0764.
N2-benzylidene-hexafluoro-2,7-diaminonaphthalene (3b) was synthesized from diamine 3a (0.64 g), yield 0.2 g (29%). Fusible yellow-orange powder. UV-vis, λmax/nm (D): 266 (0.43), 328 (0.13). 1Н NMR, chloroform-d, δ/ppm, J/Hz: 4.14 (br.s, 2Н, NH2), 7.47-7.55 (m, 3H, H3’, H5’, H4’), 7.95 (m, 2H, H2’, H6’), 8.65 (br.s, 1H, C=H). 19F NMR, chloroform-d, δ/ppm, J/Hz: 8.9 (m, 1F, F6), 10.4 (m, 1F, F3), 11.4 (m, 2F, F4, F5), 16.5 (dm, 1F, F8, JFF = 66), 23.4 (dm, 1F, F1, JFF = 66). Found: m/z 354.0589 [M]+. C17H8N2F6. Calculated: M = 354.0592.
Reaction of polyfluorinated arylenediamines with hexafluoronaphthoquinone 7 (a typical procedure). Arylenediamine (1 mmol) was dissolved in THF (5-7 ml) and quinone 7 (0.27 g, 1 mmol) was added with stirring, after dissolving of which triethylamine (0.10 g, 1 mmol) was added dropwise. The mixture obtained was stirred at 45-50 °С in an argon atmosphere for 20 h. On completion, water (20 ml) was added and products were extracted with diethyl ether (3x20 ml); the combined ether extract was washed with water and dried over MgSO4. Solvent was evaporated under reduced pressure. The product was isolated by column chromatography on SiO2, eluent hexane–diethyl ether (5:1).
2-(3’-amino-2’,5’-difluorophenylamino)-pentafluoro-1,4-dihydronaphthalene-1,4-dione (1c) was synthesized from diamine 1a (0.14 g), yield 0.22 g (57%). Mp 198-201 °С. UV-vis, λmax/nm (logε): 228 (4.4), 270 (4.3), 333 (3.6), 453 (3.5). FTIR, ν/cm-1: 3462, 3371, 3232 (N-H), 3086 (Сar-Н), 1682 (C=O). 1Н NMR, acetone-d6, δ/ppm, J/Hz: 5.12 (br.s, 2Н, NH2), 6.32 (ddd, 1Н, Н4', JFH = 9, JFH = 6, JHH = 3), 6.48 (ddd, 1Н, Н6', JFH = 10, JFH = 7, JHH = 3), 7.93 (br.s, 1Н, NH). 19F NMR, acetone-d6, δ/ppm, J/Hz: 11.6 (ddd, 1F, F2’, JFF = 12, JFH = 7, JFH = 6), 15.0, 17.8 (both m, in 1F, F6, F7), 21.7 (br.s, 1F, F2), 23.4, 25.0 (both m, in 1F, F5, F8), 44.4 (ddd, 1F, F5', JFF = 12, JFH = 10, JFH = 9). Found: m/z 390.0232 [M]+. C16H5O2N2F7. Calculated: M = 390.0234.
2-(3’-amino-2’,4’,5’-trifluorophenylamino)-pentafluoro-1,4-dihydronaphthalene-1,4-dione (4c) was synthesized from diamine 4a (0.16 g), yield 0.21 g (52%). Mp 179-180 °С. UV-vis, λmax/nm (logε): 228 (4.4), 269 (4.4), 335 (3.6), 457 (3.6). FTIR, ν/cm-1: 3417, 3367, 3259 (NH2), 3081 (Сar-Н), 1691 (C=O). 1Н NMR, acetone-d6, δ/ppm, J/Hz: 5.22 (br.s, 2Н, NH2), 6.58 (ddd, 1Н, Н6', JFH = 11, JFH ~8, JFH ~8), 7.97 (br.s, 1Н, NH). 19F NMR, acetone-d6, δ/ppm, J/Hz: 4.1 (ddd, 1F, F4’, JFF = 21, JFF =11, JFH = 8), 15.0, 17.9 (both m, in 1F, F6, F7), 17.6 (ddd, 1F, F2', JFF ~11, JFF ~11, JFH = 8), 18.9 (ddd, 1F, F5', JFF = 21, JFF ~11, JFH ~11), 19.8 (br.s, 1F, F2), 23.4, 25.0 (both m, in 1F, F5, F8). Found: m/z 408.0145 [M]+. C16H4O2N2F8. Calculated: M = 408.0140.
Analytical and spectral studies were performed in the Multi-Access Chemical Research Center SB RAS.
References
- Wozniak A.I., Yegorov A.S., Ivanov, V.S., Igumnov S.M., Tcarkova K.V. Recent progress in synthesis of fluorine containing monomers for polyimides // J. Fluorine Chem. 2015, vol. 180, p. 45–54; Fujiwara, T., O’Hagan, D. Successful fluorine-containing herbicide agrochemicals // J. Fluorine Chem. 2014, V.167, p. 16–29; Muller, K., Faeh, C., Diederich, F. Fluorine in Pharmaceuticals: Looking Beyond Intuition // Science 2007, V. 317, p. 1881–1886.
- Ostrowska, M., Fritsky, I.O., Gumienna-Kontecka, E., Pavlishchuk, A.V. Metallacrown-based compounds: Applications in catalysis, luminescence, molecular magnetism, and adsorption // Coord. Chem. Rev. 2016, vol. 327-328, p. 304-332; Lacroix, P.G., Malfant, I., Lepetit, C. // Second-order nonlinear optics in coordination chemistry: An opendoor towards multi-functional materials and molecular switches // Coord. Chem. Rev. 2016, vol. 308, p. 381–394; Braga, D., Grepioni, F., Maini, L., d’Agostino, S. Making crystals with a purpose; a journey in crystal engineering at the University of Bologna. IUCrJ 2017, vol. 4, p. 369–379; Busseron, E., Ruff, Y., Moulin, E., Giuseppone, N. Supramolecular self-assemblies as functional nanomaterials // Nanoscale 2013, vol. 5, p. 7098–7140; Chi, Z., Zhang, X., Xu, B., Zhou, X., Ma, C., Zhang, Y., Liu, S., Xu, J. Recent advances in organic mechanofluorochromic materials // Chem. Soc. Rev. 2012, vol. 41, p. 3878−3896.
- Yuan, M.-S., Wang, D.-E., Xue, P., Wang, W., Wang, J.-C., Tu, Q., Liu, Z., Liu, Y., Zhang, Y., Wang, J. Fluorenone Organic Crystals: Two-Color Luminescence Switching and Reversible Phase Transformations between π−π Stacking-Directed Packing and Hydrogen Bond-Directed Packing // Chem. Mater. 2014, vol. 26, p. 2467−2477; Ushakov, E.N., Martyanov, T.P., Vedernikov, A.I., Pikalov, O.V., Efremova, A.A., Kuz'mina, L.G., Howard, J.A.K., Alfimov, M.V., Gromov, S.P. Self-assembly through hydrogen bonding and photochemical properties of supramolecular complexes of bis(18-crown-6)stilbene with alkanediammonium ions // J. Photochem. Photobiol. A – Chem. 2017, vol. 340, p. 80−87; Hinoue, T., Miyata, M., Hisaki, I., Tohnai, N. Guest-Responsive Fluorescence of Inclusion Crystals with p-Stacked Supramolecular Beads // Angew. Chem. Int. Ed. 2012, vol. 51, p. 155–158.
- Vaganova, T.A., Gatilov, Yu.V., Malykhin, E.V. Crystal associates of 18-crown-6 and polyfluoro(het)arylenediamines: structure, properties, and selectivity of formation // Russ. Chem. Bull. Int. Ed. 2015, vol. 64, p. 1746−1756; Vaganova, T.A., Gatilov, Yu.V., Malykhin, S.E., Pishchur, D.P., Larichev, Yu.V., Rodionov, V.I., Malykhin, E.V. Design and supramolecular structure of crystal associates of polyfluoroarylenediamines and 18-crown-6 (2:1) // J. Mol. Struct. 2017, vol. 1133, p. 122–134.
- Vaganova, T.A., Gatilov, Yu.V., Pishchur, D.P., Chuikov, I.P., Malykhin, E.V. Controlled self-assembly of π-stacked/H-bonded 1D crystal structures from polyfluorinated arylamines and 18-crown-6 (2 : 1). Associate vs. co-former fluorescence properties // CrystEngComm 2018, vol. 20, 807–817.
- Vaganova, T.A., Kusov, S.Z., Rodionov, V.I., Shundrina, I.K., Malykhin, E.V. Selective mono- and diamination of polyfluorinated benzenes and pyridines with liquid ammonia // Russ. Chem. Bull. Int. Ed., vol. 56, p. 2239–2246.
- Vaganova, T.A., Kusov, S.Z., Rodionov, V.I., Shundrina, I.K., Sal’nikov, G.E., Mamatyuk, V.I., Malykhin, E.V. Amination of octafluoronaphthalene in liquid ammonia. 2,6- and 2,7-Diaminohexafluoronaphthalenes selective preparation // J. Fluorine Chem. 2008, vol. 129, p. 253–260.
- Rodionov, V.I., Vaganova, T.A., Malykhin, E.V. Selective mono- and diamination of some poly-halogenbenzenes in anhydrous ammonia // J. Fluorine Chem. 2015, vol. 180, p. 98–102.
- Moussallem, C., Allain, M., Mallet, C., Gohier, F., Frère, P. Fluorine–fluorine type II versus πF–π stacking interactions in the supramolecular organizations of extended thiophene derivatives end capped by imino-perfluorophenyl units // J. Fluorine Chem. 2015, vol. 178, 34–39; Yeap, G.Y., Ha, S.T., Boey, P.L., Mahmood, W. A. K., Ito, M. M., Youhei, Y. Synthesis and characterization of some new mesogenic schief base esters N-[4-(4-n-hexadecanoyloxybenzoyloxy)-benzylidene]-4-substituted anilines // Mol. Cryst. Liq. Cryst. 2006, vol. 452, p. 73–90; Collings, P.J., Hird, M. Introduction to Liquid Crystals Chemistry and Physics // Philadelphia, Taylor & Francis Ltd, 1997, 298 p.; Bilgin-Eran, B., Yörür, Ç., Tschierske, C., Prehm, M., Baumeister, U. Liquid crystals based on semiperfluorinated imines and salicylaldimato metal complexes. A comparative study of alkyl, alkoxy and polyether substituents // J. Mater. Chem. 2007, vol. 17, p. 2319–2328; Zhu, S., Zhu, S., Jin, G., Li, Z. Strong phenyl–perfluorophenyl π–π stacking and C–H···F–C hydrogen bonding interactions in the crystals of the corresponding aromatic aldimines // Tetrahedron Lett. 2005, vol. 46, p. 2713–2716.
- Nain-Perez, A., Barbosa, L.C.A., Picanco, M.C., Giberti, S., Forlani, G. Amino-substituted para-Benzoquinones as Potential Herbicides // Chem. Biodiversity 2016, 13(8), 1008–1017.
- Goryunov, L.I., Troshkova, N.M., Nevinskii, G.A., Shteingarts, V.D. Synthesis of 2-Aminopentafluoro-1,4-naphthoquinone Derivatives // Russ. J. Org. Chemistry, 2009, vol. 45, p. 835–841.
- Barbosa, L.C.A., Pereira, U.A., Maltha, C.R.A., Teixeira, R.R., Moreira Valente, V.M., Oliveira Ferreira, J.R., Costa-Lotufo, L. V., Moraes, M.O., Pessoa, C. Synthesis and Biological Evaluation of 2,5-Bis(alkylamino)-1,4-benzoquinones // Molecules 2010, vol. 15, p. 5629–5643; Park, H., Carr, B.I., Li, M., Ham, S.W. Fluorinated NSC as a Cdc25 inhibitor // Bioorg. Med. Chem. Lett. 2007, vol. 17, p. 2351–2354.
- The Quinonoid Compounds (1988): Part 1, vol. 1. Editors(s): Patai, S., Rappoport, Z. John Wiley & Sons Ltd. 1988, 878 p. DOI:10.1002/9780470772119.
- Bayen, S., Barooah, N., Sarma, R. J., Sen, T. K., Karmakar, A., Baruah, J.B. Synthesis, structure and electrochemical properties of 2,5-bis(alkyl/arylamino)1,4-benzoquinones and 2-arylamino-1,4-naphthoquinones// Dyes Pigm. 2007, vol. 75, p. 770–775.
- Ando, S., Matsuura, T., Sasaki, S. in Hougham G. (Ed), Fluoropolymers 2: Properties // New York, Kluwer Academic/Plenum Publishers, 1999, p. 277–303.
- Li, A., Bin, X., Zhu, S. Synthesis of N-pentafluorophenyl aromatic aldimines C6F5N= CHAr and an X-ray structure analysis of N-pentafluorophenyl-4-methylphenyl aldimine, C6F5N=CHC6H4CH3-4 // J. Fluor. Chem. 1994, vol. 68, p. 145–148.
- Sasaki, I., Amou, T., Ito, H., Ishiyama, T., Iridium-catalyzed ortho-C–H borylation of aromatic aldimines derived from pentafluoroaniline with bis(pinacolate)diboron // Org. Biomol. Chem. 2014, vol. 12, p. 2041-2044.
- Casey, C.P., Johnson, J.B. Isomerization and Deuterium Scrambling Evidence for a Change in the Rate-Limiting Step during Imine Hydrogenation by Shvo's Hydroxycyclopentadienyl Ruthenium Hydride // J. Am. Chem. Soc. 2005, vol. 127, p. 1883–1894.
- Gerasimowa, T.N., Semikolenova, N.V.; Fokin, E.P. Some reactions of polyfluoro-substituted N-benzylidenanilines // Izv. SO AN SSSR, Ser. Khim. Nauk 1977, vol. 2; p. 142–145 (in Russian). CA 1977, 87:134160.
- Murza, M.M.; Tataurov, G.P.; Popova, L.I.; Svetkin, Yu.V. Liquid-crystalline substances. Fluorine-containing aromatic azomethines // J. Org. Chemistry USSR (English Translation) 1977, vol. 13, p. 961. CA 1977, vol. 87, 134286.
- Bogachev, A.A., Kobrina, L.S., Yakobson, G G. The interaction of polyfluoroaromatic compounds with oxygen containing radicals // J. Fluorine Chem. 1985, vol. 29, p. 143.
- Yakobson, G.G., Shteigarts, V.D., Vorozhtsov-Jr, N.N. Reaction of octafluoronaphthalene with nitric acid // Zhurn. Vsesoyuz. Khim. O-va im. D.I. Mendeleeva 1964, vol. 9, N 6, p. 702–704 (in Russian). CA. 1965, vol. 62, 9078b.
Recommended for publication by Prof. V.E. Platonov
Fluorine Notes, 2018, 119, 5-6
