Received: June 2018
DOI 10.17677/fn20714807.2018.04.01
Fluorine Notes, 2018, 119, 1-2
Synthesis and properties of CF2X-substituted 4-methyl-2-hydroxy(chloro)pyrimidines
V. I. Dyachenko
A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, V-334,
GSP-1, 119991 Moscow, Russia
e-mail: vic-d.60@mail.ru
Abstract: 4-Trifluoromethyl-6-methylpyrimidine-2-ol (3a) and 4-difluoromethyl-6-methylpyrimidine-2-ol (3b) were synthesized by the condensation of 1,1,1-trifluoropentane-2,4-dione (1а) and 1,1-difluoropentane-2,4-dione (1b) correspondently with urea (2) in CH3CO2H under reflux in high yields. 2-Chloro-4-trifluoromethyl-6-methylpyrimidine (4a) and 2-chloro-4-difluoromethyl-6-methylpyrimidine (4b) were obtained by the reaction of (3a) and (3b) correspondently with POCl3.
Keywords:1,1,1-trifluoropentane-2,4-dione, 1,1-difluoropentane-2,4-dione, 4-trifluoromethyl-6-methylpyrimidine-2-ol, 4-difluoromethyl-6-methylpyrimidine-2-ol, 2-chloro-4-methyl-6-trifluoromethyl-pyrimidine, 2-chloro-4-difluoromethyl-6-methylpyrimidine.
Pyrimidine bases, which are included in DNA composition, along with purines, are universal chemical base for recording, holding and transferring of information about living organisms construction [1]. Scientists found out the way how to influence the speed of processes occurring in cells by partially changing nucleotides structure without its mispairing. «Masking effect», connected with atoms H and F proportions, is the most frequently used method for that purpose [15].
Floxuridine, 5-fluorouracil, tegafur, gemcitabine and capecitabine able to integrate into the structure of DNA and RNA replacing natural nucleotides slow down the activity and division of cancer cells and thus found application in medical practice. (Pic.1).
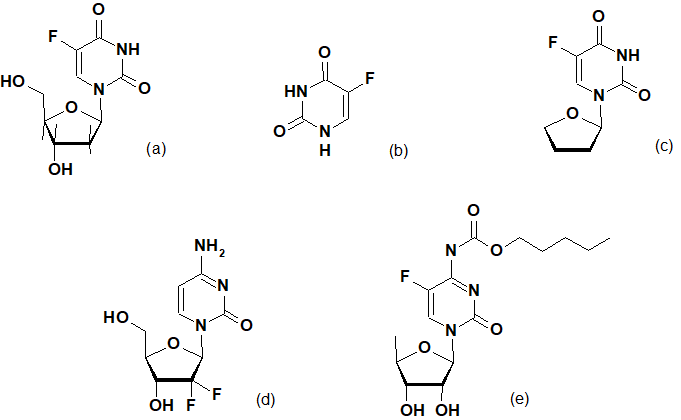
Pic.1. Synthetic fluorine-containing pyrimidines: floxuridine (а), fluorouracil (b), tegafur (c), gemcitabine (d) and capecitabine (e)
СF3-Substituted pyrimidines are also important for sell metabolism. 4-Trifluoromethyl-6-methylpyrimidine-2-ol (3а) derivatives are chemokine receptor antagonists [2]. Vinylpyrimidones obtained on its base exhibit antiviral activity [3]. Chiral 4-trifluoromethyl-6-methylpyrimidine 2-phenoxypropionates are suggested as herbicides [4]. Other its analogues are effective fungicides [5]. Substituted piperazines containing the structure of 4-trifluoromethyl-6-methylpyrimidine are patented as modulators of Х-receptors of liver [6], and 1,2,5-oxadiazepam derivatives are used for treatment of depression, dementia and also Alzheimer’s disease [7].
The goal of this study is a search of effective methods for the preparation of 4-tri(di)fluoromethyl-6-methylpyrimidine-2-oles 3а,b and the synthesis of its 2-chlorine-containing analogues 4а,b (Scheme 1). 4-Trifluoromethyl-6-methylpurimidine-2-ol 3а was obtained for the first time in 1941 by refluxing of 1,1,1-trifluoropentane-2,4-dione 1а and urea 2 in ethanol in the presence of Н2SO4 for 24-48 hours with the yield of 87% [8, 9].
Scheme 1.
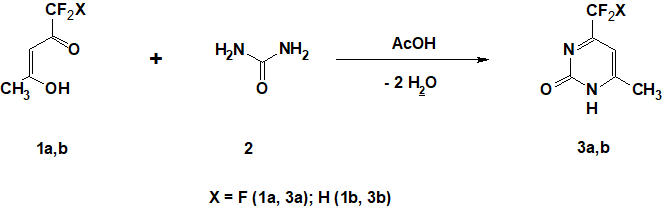
Later it was stated that in these conditions the authors [2, 3] succeed in preparation of pyrimidine 3а only in 15 and 20% yield correspondingly. Pyrimidine’s 3а yield also was not higher than 19% by the reaction of pentandione 1а with cyanamide [10]. According to the literature data [11, 13], condensation of urea with other 1,3-diketones containing one fluorinated substituent by refluxing for 20 hours in ethanol in the presence of concentrated HCl, leads to the pyrimidine-2-ol with the yield only of 34%.
We found out that diketone 1а reacts with urea 2 at "green chemistry" conditions – under reflux in glacial acetic acid for 1-1,5 hours. Spectroscopically pure 3а was obtained by crystallization from water with the yield higher than 90%. In the same conditions, using the condensation of 1,1-difluoropentane-2,4-dione 1b and urea, we obtained 4-difluoromethyl-6-methylpyrimidine-2-ol 3b for the first time in 83% yield . (Scheme 1). The results obtained show that low yields of pyrimidine 3а published earlier was due to the reaction temperature which was not high enough. In fact, instead of boiling in acetic acid offered by us (boiling temperature 118°С), in all previous studies [2, 3, 11-13] condensation of diketone 1а and urea 2 was held in ethanol (boiling temperature 78°С).Not paying due attention to the effect of temperature on the reaction rate, the authors focused on acid catalysis(H2SO4, HCl, р-СH3-C6H4-SO3H), which, apparently, does not play a decisive role in these transformations. We found that 3а formation occurs with comparable speed and with the same yield by refluxing of 1а and 2 in ethylene glycol monomethyl ether (its boiling temperature 124°С, that is near to bp of acetic acid), as in acetic acid, while ethylene glycol monomethyl ether is not an acid.
In order to expand the synthetic capabilities of pyrimidines containing CF2X- function, we studied nucleophilic substitution of the the ОН-group for chlorine in 3а,b. Earlier it was noted [2] that when refluxing 3а with РОСl3 the maximum yield of chloropyrimidine 4а was 37%. The same result [14] was obtained in the reaction 3а with phosphorus oxide in the presence of DMAP at 95°С.
Scheme 2.
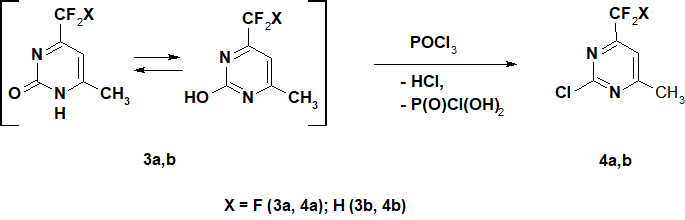
We have shown that when refluxing with РОСl3 for 11 hours 3а turns into the corresponding 2-chlorinated derivative 4а with a high conversion (Scheme 2). After isolation of the product from the reaction mixture and vacuum distillation, 2-chloro-4-methyl-6-trifluoromethylpyrimidine 4a was obtained in a yield of 76.5%. According to gas chromatography data its purity was 99%. 2-Chloro-4-difluoromethyl-6-methylpyrimidine 4b was obtained for the first time from the compound 3b, using the same method, in 74.4% yield.
Presumably, low yields of chloropyrimidine 4а obtained earlier [2, 14] was due to the high reaction ability of chlorine in 2-position and peculiarities of its isolation.
Experimental
1H, 19F NMR spectra were recorded in DMSO-d6 and CDCl3 using «Bruker Avanse 400» spectrometer with operating frequency 400.13 MHz, 376.5 MHz correspondingly. Chemical shifts for 1Н are given according to ТMS (internal standard), 19F according to CF3CO2H – external standard. Spin-spin interaction constants are given in MHz. Mass-spectrum are recorded on quadrupolar spectrometer Finnigan MAT INCOS 50 (direct input, ionization energy 70 eV).
4-Trifluoromethyl-6-methylpyrimidine-2-ol (3а) 4.8 g (80 mmol) of urea 2 and 12.8 g (80 mmol) of 1,1,1-trifluoroacetylacetone 1а during 1 hour were being reflux in 48 ml of glacial acetic acid in a glass bulb equipped with a reflux condenser, dropping funnel and magnetic stirrer. The reaction mixture was evaporated on a rotor evaporator and the crude product 3а was crystallized from water, filtered, dried on air, then in vacuum desiccator over P2O5. White crystalline pyrimidine 3а (13 g) was obtained, melting point 181-182°С (water). Yield 91%. Rf=0.4 (ethylacetate). 1H NMR spectra,(δ, ppm): 12.76 (br. s., 1Н, OH); 6.77 (s, 1Н, Het); 2.35 (s, 3Н, СН3). 19F NMR spectra, (δ, ppm): 7.85 (s). Mass-spectra, m/z): 178 [M]+. Found, %: С, 40.33; Н, 2.71; F, 31.78. С6H5F3N2O. Calculated, %: C 40.46; Н 2.83; F 32.00.
4-Difluoromethyl-6-methylpyrimidine-2-ol (3b) was obtained using the same method as 3а from 1.2 g (20 mmol) of urea 2 and 3.2 g (20 mmol) of 1,1-difluoroacetylacetone 1b in 12 ml of glacial acetic acid for 1.5 hour. The solvent was evaporated on a rotor evaporator, and the oil obtained was treated with ether while mixing. The solid residuum was cooled to -15°С, ground while mixing and filtered. The product was washed with ether and dried on filter, 2.7 g of amorphous substance 3b were obtained. Yield 83%, melting point 130-131°С (tert-ВuОН), Rf=0.5 (acetone-ethyl acetate=1:3). 1Н NMR spectra, (δ, ppm; J, MHz): 12.36 (br. s, 1Н, ОН); 6.60 (t, 1Н, СF2H, 2J=52); 6.53 (s, 1Н, Het); 2.30 (s, 3Н, СН3). 19F NMR spectra, (δ, ppm; J, MHz): 120.00 (d, 2F, CF2H, 2J=52). Mass-spectra, m/z: 160 [M]+. Found, %: С, 45.00; Н, 3.63; F, 23.31. С6H6F2N2O. Calculated, %: C, 45.01; Н, 3.78; F, 23.73.
2-Chloro-4-methyl-6-trifluoromethylpyrimidine (4a) In a glass bulb equipped with a reflux condenser and magnetic stirrer with heating 14.2 g (0.08 mmol) of 3а and 90 g of POCl3 were refluxed for 11 hours. The reflux condenser was changed to descending condenser and the excess of POCl3 was distilled at a normal pressure. After this, from the stillage residue chloropyrimidine 4a and other volatile compounds were distilled with water jet pump (15 mm Hg). For 4a purification crude chloropyrimidine 4a with POCl3 admixture was added drop by drop in a 1 l glass with. 200 ml of cold water. At the same time concentrated soda solution was added in the reaction mixture. 4a was extracted from the reaction mixture with benzene (50 ml х 3 times), organic solution was dried with calcium chloride and filtered through silica gel. After removing of the solvent 14 g of thin oil were obtained. By its distillation with Wurtz cap 12 g (76.5%) of chloropyrimidine 4a was obtained, boiling temperature 69-70°С/11 mm Hg, nD20 1.4450. Its purity is 99%. 1Н NMR spectra, (δ, ppm): 7.46 (s, 1Н, Het); 2.67 (s, 3Н, CН3). 19F NMR spectra, (δ, ppm): 6.78 (s, 3F, CF3). Mass-spectra, m/z: 196 [M]+. Found, %: C, 36.38; Н, 2.15; F, 28.69. С6H4СlF3N2. Calculated, %: C, 36.66; Н, 2.05; F, 29.00.
2-Chloro-4-difluoromethyl-6-methylpyrimidine (4b) was obtained using the same method as 4а by refluxing 14.4 g (0.09 mmol) of 3b and 105 g of POCl3 for 12 hours. After the crude product distillation under vacuum with Wurtz cap 11.9 g of 4b was obtained in the form of transparent oil liquid with the boiling point 84-85°С/11 mm Hg. Yield 74.4 %. n20D = 1.4800. 1Н NMR spectra, (δ, ppm; J, MHz): 7.42 (s, 1Н, Het); 6.48 (t, 1Н, СF2H, J=52); 2.63 (s, 3Н, СН3). 19F NMR spectra, (δ, ppm; J, MHz): -120.00 (d, 2F, CF2H, 2J=52). Mass-spectra, m/z: 178 [M]+. Found, %: С, 39.99; Н, 3.01; F, 20.98. С6H5СlF2N2. Calculated, %: C, 40,36; Н, 2.82; F, 21.28.
Conclusion
Eco-friendly, low energy-consuming method of 4-CF2X-substituted 6-methylpyrimidine-2-ol 3а,b preparation was found. Using this method, 4-difluoromethyl-6-methylpyrimidine-2-ol 3b was obtained for the first time.
2-chloro-4-methyl-6-trifluoromethylpyrimidine 4a and 2-chloro-4-difluoromethyl-6-methylpyrimidine 4b were obtained with the yields higher than 74% for the first time by refluxing of pyrimidine-2-ol 3а,b with phosphorus chlorooxide.
Acnowledgement
The author is thankful for recording of 1Н, 19F NMR, mass-spectra and for elemental analysis to the Center of Structural Research of A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Moscow.
The present work has been executed due to financial support of Russian Foundation for Basic Research (RFBR) (project 16-29-05334-ofi-m)
Literature
- A. Kornberg, T. A. Baker. Chapter 15: The replication mechanisms and operations // DNA replication. — Sausalito, Calif.: University Science Books, 2005.
- C. G. Kenneth, R. Prakash, S. Francois. Y. Qing, G. Shomir, WO 2006071875, 2006.
- A. Missio, T. Herget, A. Aschenbrenner, B. Kramer, J. Leban, K. Wolf, WO 2004016271, 2004.
- T. Haiyang, H. Tonghui, Z. Aidong, H. Changjian, Patent China № 101613322, 2009.
- L. Long, L. Qiang, T. Qinghong, L. Xinting, D. Ming, L. Shimeng, S. Juan, Patent China № 103965117, 2014.
- D. A. Claremon, C. Dong, Y. Fan, K. Leftheris, S. D. Lotesta, S. B. Singh, C. M. Tice, W. Zhao, Y. Zheng, L. Zhuang, WO 2016022521, 2016.
- T. Kamei, Y. Arikawa, T. Ohashi, T. Imaeda, I. Fujimori, T. Miki, J. Yonemori, Y. Oguro, T. Sugimoto, M. Seto, G. Nishida, M. Kamata, H. Imoto, WO 201617124, 2016.
- J. J. Donleavy, M. A. Kise, Org. Synth. 1, 1941.
- J. C. Sloop, C. L. Bumgardner, W. D. Loehle, J. Fluor. Chem., 2002, 118-135.
- A. Miller, J. Org. Chem., 1984, 49, 21, 4072, DOI:10.1021/jo00195a043.
- A. Kreutzberger, U. H. Tesch, Chem. Ber., 1976, 109, 10, 3255.
- S. Dilli,. K. Robards, Austral. J. Chem., 1978, 31, 8, 1833.
- Ya. V. Burgart, O. S. Kuzueva, M. V. Pryadeina, C. O. Kappe and V. I. Saloutin, Russ. J. Org. Chem., 2001, 37, 6, 869. (Zh. Org. Khim., 2001, 37, 6, 915).
- H. Tu, T. Huang, A. Zhang, C. Hou, Patent China № 101613322, 2009.
- H-J. Böhm, D. Banner, S. Bendels, M. Kansy, B. Kuhn, K. Müller, U. Obst-Sander, M. Stahl, ChemBioChem 2004, 5, 637, DOI: 10.1002/cbic.200301023.
Recommended for publication by Prof. S.R. Sterlin
Fluorine Notes, 2018, 119, 1-2
