Received: April 2018
DOI 10.17677/fn20714807.2018.03.03
Fluorine Notes, 2018, 118, 5-6
Recovery of Zirconium From Alumina Production Red Muds
L.А. Pasechnik, I.S. Medyankina, V.М. Skachkov, N.А. Sabnirzyanov, I.N. Pyagai, S.P. Yatsenko
Federal State Government-Financed Institution of Science Institute of Solid State Chemistry
UB RAS,
91, Pervomaiaskya st., Ekaterinburg, 620990 (Russia)
е-mail: pasechnik@ihim.uran.ru,
vms@weburg.me
Abstract:The procedure of zirconium fluoride salts recovery from alumina production wastes – red muds (RM) – has been described. The scientific foundations of carbonation leaching of zirconium with the use of kiln waste gases have been developed. A basic manufacturing sequence of obtaining of target products has been elaborated.
Keywords: Red mud, recovery, ecology, zirconium, soda-alkaline treatment, hexafluozirconate
Introduction
Rare metals are of special strategic importance for modern industrial production. The world’s largest zirconium producers (with approximate volume of output, t/year) are AREVA NP, France (2200); ТVEL JSC, Russia (900); Westinghouse, US (800); Teledyne Wah Chang, US (1000); and NFC, India (250) [1]. According to data [2], the world output of zirconium concentrates has been declining for several years (~5% drop in 2015 compared with 2014 and another 3.9% drop in 2016 compared with 2015), and only in 2017 some growth of production was outlined, but the output does not keep pace with steadily increasing consumption [3], which indicates that it is necessary to search for new sources of zirconium.
Some rare metals are accumulated as by-products of mining of more widespread ores. From this standpoint, red muds (RM) – bauxites to alumina processing wastes – are sources of a whole series of valuable metals. Recently, scientists and technologists paid attention to the carbonation technology of RM processing as the most promising route aimed first of all at scandium recovery [4-6]; however, in spite of numerous studies on sludge treatment, there is not a single factory producing rare metals from such wastes. A considerable amount of scandium, yttrium, lanthanides etc., including zirconium, which, like titanium, does not receive due attention in the development of technologies, go to waste with slurry. Considering the large scale of alumina production, more than 1000 t of zirconium oxide are annually dumped only at each of the Ural alumina plant (UAZ-SUAL JSC and BAZ-SUAL JSC). Investigations into recovery of valuable components from red muds are being carried out at the ISSC UB RAS, the conditions for obtaining of different types of products are being worked out at the experimental-industrial production area located on the territory of the alumina plant BAZ-SUAL JSC, and the corresponding equipment is being selected [7-10].
Experimental
Figure 2 shows a flow sheet of alumina production red mud processing realized at this research stage on the experimental-industrial production area Tekhnogoriya JSC (Fig. 1).

Fig. 1. A part of the experimental-industrial plant of the red mud processing unit.
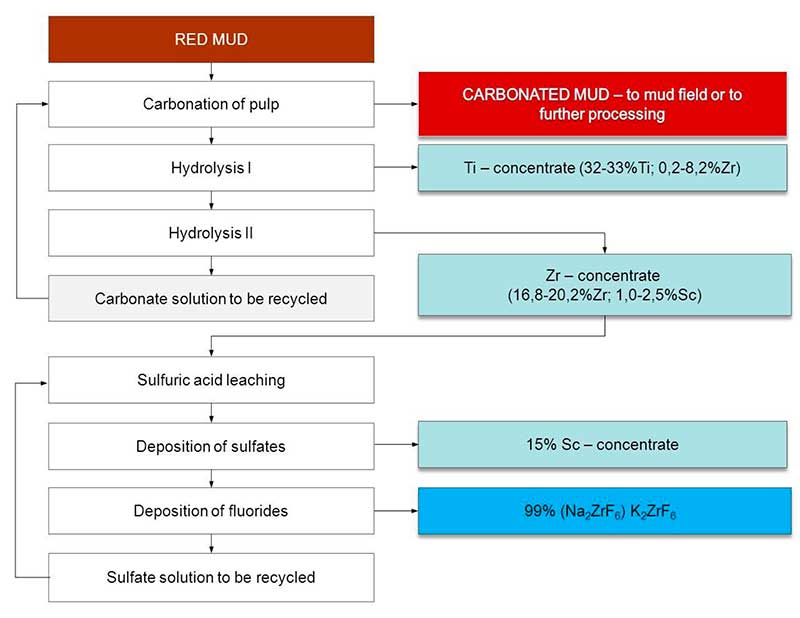
Fig. 2. The flow sheet of the soda-alkaline technology for alumina production red mud processing.
The key process in this scheme is carbonation, which is based on the ability of transition metals to form carbonate complexes. Sintering kiln gases are used as a source of CO2 (Table 1).
After removal of dust, the kiln gases are forcedly pumped through the RM pulp containing a sufficient amount of alkali that interacts with carbon dioxide:
2NaOH + H2CO3 = Na2CO3 + 2H2O;
Na2CO3 + H2CO3 = 2NaHCO3.
The process is carried out at a temperature of 505°С for several days (< 3) with multiple (< 5) carbonate-hydrocarbonate treatment of new mud batches with carbon dioxide. The concentration of NaHCO3 at the end of the process is in the range of 80-110 g/dm3.
Table 1. Characteristics of sintering kiln gases.
|
Kiln No. |
Volume of waste gases, m3/h |
Concentration of some components at outlet from kiln, wt.% |
Temperature of kiln gases, С |
Remoteness from production area, m |
||
|
СО2 |
О2 |
Solid particles, g/m3 |
||||
|
1. |
102337 |
18.4 |
1.2 |
0.03 |
65 |
150 |
|
2. |
115489 |
18.5 |
1.1 |
0.06 |
70 |
152 |
|
3. |
99546 |
18.2 |
1.1 |
0.10 |
70 |
155 |
|
4. |
74614 |
18.2 |
1.4 |
0.08 |
75 |
160 |
|
5. |
79553 |
18.0 |
1.7 |
0.09 |
65 |
170 |
Metal hydroxides, contained in RM, interact with sodium hydrocarbonate giving rise to double basic carbonates of the type Na2O(Al,Ga)2O3∙2CO2∙nH2O and soluble complexes of the composition [11] [UO2(CO3)3]4- and [UO2(CO3)2∙2H2O]2-. Titanium(IV) in alkaline medium forms hydrated titanates Na2Ti5O11∙10H2O, Na2Ti3O7∙7H2O and Na2Ti2O5∙nH2O. The transfer of zirconium(IV) into solution during carbonation of RM pulp is due to isomorphic substitution of titanium(IV) in Na4[(Zr,Ti)(CO3)4]∙nH2O-type complexes. The composition of initial RM and produced carbonate solution, as well as the products of hydrolysis are presented in Table 2.
Table 2. The composition of red mud, solution after carbonation and products of hydrolysis I and II.
|
Element |
Zr |
Ti |
Sc |
Fe |
Ca |
Si |
|
RM, wt.% |
0.064 |
2.7 |
0.012 |
29.1 |
8.7 |
4.6 |
|
Carbonate solution, mg/dm3 |
44.5 |
25.0 |
5.4 |
3.4 |
15.0 |
1,0 |
|
Deposit of hydrolysis I, wt.% |
8.0 |
32.5 |
0.015 |
27.5 |
3.6 |
2.8 |
|
Deposit of hydrolysis II, wt.% |
20.2 |
1.7 |
1.9 |
1.6 |
1.5 |
3.1 |
As a result of carbonation, greenhouse gases are absorbed, and рН of RM pulp decreases from 10-12 to 8-9, which considerably reduces the environmental impact.
For separate extraction of titanium(IV) and zirconium(IV), double hydrolysis of carbonate solutions is carried out. During hydrolysis I, the temperature of the solution is raised to 80°С, caustic soda is introduced to рН=10-10.5, and as a result of decomposition of carbonate complexes a deposit is formed (Table 2), in which titanium(IV) hydroxide is chiefly concentrated.
At the stage of hydrolysis II, the temperature is raised to 95-100°С, and рН of solution – to 12, thus leading to the decomposition of the rest carbonate complexes, the process of complete decomposition of complexes takes 5-6 h. After filtration, the solution is returned to the stage of carbonation, and the solid deposit (see its composition in Table 2) is leached with sulfuric acid. The concentration of zirconium(IV) in the produced sulfate solutions may be as much as 40 g/dm3. The composition of the solutions breaking down zirconium(IV) concentrate and of insoluble residue averaged over several experiments is presented in Table 3.
Table 3. The composition of solution and insoluble residue after breakdown of primary concentrate.
|
Element |
Zr |
Ti |
Na |
Sc |
Fe |
Al |
Ca |
Si |
|
Solution, g/dm3 |
18.5 |
3.5 |
13.8 |
2.0 |
2.2 |
0.5 |
0.4 |
0.05 |
|
Residue, wt.% |
0.2 |
0.5 |
0.02 |
0.01 |
8.2 |
1.5 |
13.4 |
28.0 |
To remove scandium(III) from solution, it is salted out by sulfuric acid and a salting-out reagent. The subsequent operation after removal of scandium(III) is deposition of zirconium(IV) in the form of fluoride salts or fluorosulfates (Zr(SO3F)4) [12]. It was established experimentally that after the introduction of a solution of a mixture of alkaline metal fluoride and hydrofluoric acid taken in a certain ratio of components, zirconium is amply deposited from the solution (98.0-99.8%). For quantitative deposition of zirconium, we determined the optimal compositions of the alkaline metal fluoride and hydrofluoric acid mixture, as well as the acidity of the initial solution. Zirconium(IV) interacts with the reagents in the solution according to the chemical reaction:
Zr(SO4)2 + 2KF + 4HF = K2ZrF6 + 2H2SO4.
The introduction of hydrofluoric acid as a source of F— ions increases the acidity of solutions and accordingly the concentration of the reaction product H2SO4, the equilibrium being displaced towards the formation of initial substances. It was found experimentally that the synergetic effect (virtually complete deposition of zirconium(IV) at its low content in the initial solution) is achieved owing to the optimal combination of the acidity level of the initial solution and the amount of introduced mixture containing fluoride ion. For example, when the concentration of sulfuric acid in the solution is more than 400 g/dm3, the introduction of an excessive amount of the solution of (NaF+HF) mixture containing fluoride ion leads to the appearance of a considerable quantity of sodium fluoride in the zirconium product. The composition of such deposit according to XPA data (Fig. 3) is as follows: 50% Na2ZrF6·NaF and 50% NaF·HF.
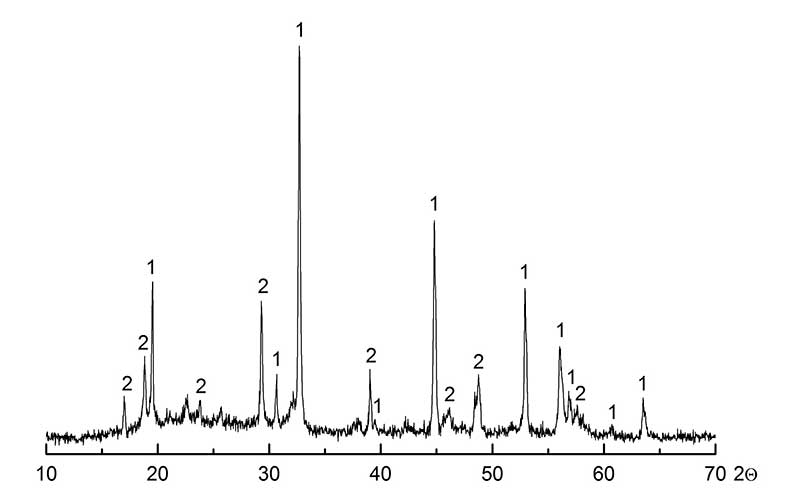
Fig. 3. The X-ray diffraction pattern of zirconium fluoride concentrate: 1 – acid sodium fluoride NaHF2; 2 – sodium fluozirconate Na3ZrF7
By varying the parameters (deposition duration, temperature, elemental and quantitative composition of alkaline metal fluoride mixture) in the experimental studies, we selected the optimal conditions of zirconium(IV) deposition from sulfate solutions. The composition of zirconium concentrate produced in several experiments on zirconium(IV) deposition is given in Table 4.
Table 4. The composition of zirconium concentrate produced from sulfate solution upon removal of scandium, wt.%.
|
Zr |
Al |
Ca |
Sc |
Fe |
Ti |
Th |
U |
|
19.7 |
0.14 |
0.15 |
0.15 |
0.25 |
0.26 |
0.002 |
0.001 |
A photomicrograph of zirconium fluoride concentrate and the energy-dispersive spectrum of a surface segment are demonstrated in Figs. 4 (а) and 4 (b), respectively.
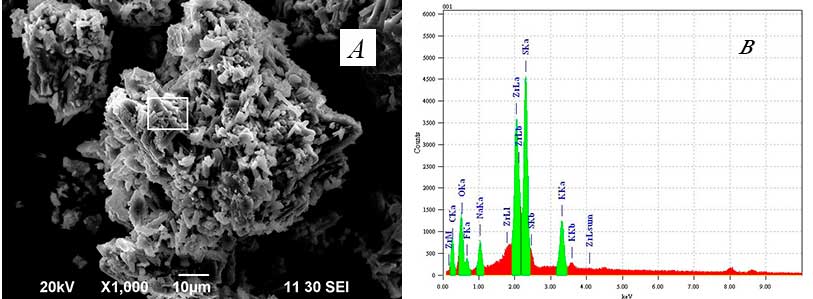
Fig. 4. Zirconium fluoride concentrate: а – electronic photomicrograph, х1000 magnification; b – energy-dispersive spectrum of a surface segment
Thus, the conditions of zirconium fluoride concentrate deposition from zirconium-containing solution with sulfuric acid content of 10 – 400 g/dm3 were determined. For quantitative deposition, it was sufficient to use mixtures of potassium or sodium fluoride and hydrofluoric acid with the molar ratio K(Na)F : HF = 0.5 1.5 : 1 as a source of fluoride ion. The process takes place during a small heating to 40-60°С after the introduction of reagents; then the mixture is held at room temperature for 22-24 h for complete formation of deposit. Total zirconium recovery from RM by the developed technology can be as much as 60%, which gives a considerable amount of valuable metal considering the large scale of alumina production; moreover, the environmental situation around the plant is improved (absorption of carbon dioxide, reduction of рН of RM directed to sludge depository), no new production wastes are formed, and semiproducts suitable for further recycling (titanium and scandium concentrates) are obtained.
Conclusions
The studies performed provided the basis for the engineering solutions for creating an experimental-industrial production area on the territory of the alumina plant BAZ-SUAL JSC using large-size apparatuses. A soda-alkaline method of zirconium recovery from RM has been developed and tested in laboratory and semi-industrial conditions, which may considerably improve the environmental situation around alumina plants in the Urals in case of large-scale introduction of this technology. The processes of dissolution of concentrates and deposition of valuable components from acid zirconium-containing solutions by salting-out (Sc) and introduction of fluoride ions (Zr) have been worked out. By introducing KF and HF (or NaHF2) containing solution we have obtained potassium hexafluozirconate K2[ZrF6] with a low content of titanium and hafnium impurities; the main impurities of such product are sodium, potassium and sulfate ions as residues of mother solution that are easily removed by thorough washing [13].
The work was carried out in accordance with the state assignment and R&D plans of the ISSC UB RAS (No. AAAA-A16-116122810213-2).
References
- Pilipenko N.N. Production of nuclear-quality zirconium. / Pilipenko N.N. // Voprosy atomnoi nauki i tekhniki. 2008. No. 2. Series: Fizika radiatsionnykh povrezhdenii i radiatsionnoe materialovedenie (92), P. 66-72. [in Russian]
- U.S. Geological Survey. Mineral Commodity Summaries. (January 2016, 202 р. January 2017, 202 р. January 2018, 200 р.)
- Petrov I.М. Outlooks for world’s REM market development. / I.М. Petrov // Topical problems of REM production and application – 2015: International theoretical and practical conference information package. – Moscow: ОАО INSTITUT GINTSVETMET. – 2015. – P. 13-15. [in Russian]
- Imideev V.А., Aleksandrov P.V., Boboev I.R. Intensification of scandium leaching process from red muds into carbonatre-bicarbonate solutions // Tsvetnaya metallurgiya. – 2016. – No. 5. P.22. [in Russian]
- Medvedev А.S., Kirov S.S., Khairullina R.Т., Suss А.G. Carbonation leaching of scandium from red muds with the use of preliminary gassing of pulp with carbon dioxide // Tsvetnye metally. –2016. – No. 6. P. 67–73. [in Russian]
- O.V. Petrakova, A.V. Panov, S.N. Gorbachev, O.N. Milscin. Improved efficiemcy of red mud processing through scandium oxide recovery // Bauxite Residue Valorisation and Best Practices. Leuven. 5-7/10/2015. P.355-362.
- Pasechnik L.А., Pyagai I.N., Skachkov V.М., Yatsenko S.P. Recovery of rare elements from alumina production waste mud with the use of sintering kiln gases. Ekologiya i promyshlennost Rossii. 2013. No. 6. P. 36-38. [in Russian]
- 8. Pyagai, I.N.; Pasechnik, L.A.; Yatsenko, A.S.; Skachkov, V.M.; Yatsenko, S.P. // Recovery of sludge from alumina production // Russian journal of applied chemistry 2012. Vol. 85. Is. 11. Pр. 1649-1653.
- RU Patent 2478725. C22B59/00, C01F17/00, C22B3/06(2006.01). Method for production of scandium oxide. / L.А. Pasechnik, S.P. Yatsenko, I.N. Pyagai; Institute of Solid State Chemistry of the Ural Branch of the Russian Academy of Sciences. No. 2011137733/02; appl. 13.09.2011; publ. 10.04.2013. Bul. No. 10. [in Russian]
- Pasechnik L.А., А.G. Shirokova, S.P. Yatsenko, I.S. Medyankina. Concentration and treatment of rare metals in technogenic waste processing // Proceedings of Kola research center RAS. Khimiya i materialovedenie. 2015 (31). No. 5. P. 186-189. [in Russian]
- Zherin I.I., Amelina G.N. Foundations of radiochemistry, methods for recovery and separation of radioactive elements. Tomsk: Tomsk Polytechnic University Publishing house, 2009. – 196 p. [in Russian]
- Godneva М.М., Motov D.L. Chemistry of titanium subgroup. Sulfates, fluorides, fluorosulfates from aqueous media. Moscow: Nauka, 2006. 302 p. [in Russian]
- RU Patent 2623978. C22B34/14(2006.01), C22B3/44(2006.01). Method for zirconium recovery from acid aqueous solutions. / V.М. Skachkov, L.А. Pasechnik, I.N. Pyagai, L.М. Skryabneva, I.S. Medyankina, S.P. Yatsenko, N.А. Sabirzyanov; Institute of Solid State Chemistry of the Ural Branch of the Russian Academy of Sciences. No. 20161054391; appl. 17.02.2016; publ. 29.06.2017. Bul. No. 19. [in Russian]
Recommended for publication by Prof. S. M. Igumnov
Fluorine Notes, 2018, 118, 5-6
