Received: April 2018
DOI 10.17677/fn20714807.2018.03.01
Fluorine Notes, 2018, 118, 1-2
Potentiometric Micromethod for The Determination of Fluoride Ion in Natural Objects
D.B. Petrenko*,**, V.Yu. Dmitrieva*, R.A. Gazarov ***, N.V. Vasil'ev*
* Moscow Region State University, 105005, Russian Federation, Moscow, Radio Street 10а.
** Institute of Geology of Ore Deposits, Petrography, Mineralogy, and Geochemistry, Russian
Academy of Sciences, 119017, Russian Federation, Moscow, Staromonetnyi per. 35.
DBPetrenko@yandex.ru
***Gubkin Russian State University of Oil and Gas (National Research University), 119991,
Russian Federation, Moscow, Leninsky Prospect 65.
gazarov@gubkin.ru
Abstract: A simple potentiometric micromethod for the determination of fluoride ion in natural objects is described. The method allows the analysis of sample volumes starting from 0.05 cm3, does not require complex instruments to be implemented and is convenient for batch analysis.
Keywords: Fluoride, Potentiometry, Ion Selective Electrodes, Microdetermination.
Potentiometry is employed for the determination of fluoride ions in a wide range of natural and industrial objects due to its simplicity and reliability. Currently, commercially available electrodes allow the determination of fluoride ion with the lower detection limit of 0.02 mg/dm3 in samples with the volume of 5-50 cm3 [1], however when some classes of biological and natural objects are studied, only small sample volumes (≤0.5 cm3) are available. The known micromethods of fluoride ion determination use flow potentiometry and require comparatively complex instrumentation and specially designed cells [2-3]. Other methods involve either application of the solution under study onto the edge of an overturned fluoride selective electrode with the subsequent introduction of the reference electrode into the sample droplet [4, 5], or introduction of the electrodes into the studied solution in a Teflon plate well [6]. To our opinion, the abovementioned micromethods for the determination of fluoride ion have the disadvantage of requiring specifically designed apparatus, some procedures carry a significant risk of mechanical damage of the electrode membrane. It is also inconvenient, speaking of the procedures utilizing the application of sample drops onto the electrode edge, that the electrode must be removed from the holder and put back again after each measurement in order to be washed.
There are commercially available cell samples for ionometric measurements. For several decades the Gomel plant of measuring instruments has been producing a cell for micro-measurements, which is a glass with a lid, in which there are holes for installing an auxiliary electrode and an electrolytic key [7]. The electrolytic key is in the form of a cylinder with a spherical bottom, in the lower part of which there is a limited elongation with a solder in asbestos thread, which provides the connection of the electrolytic key with the auxiliary electrode. The analyzed solution and the working part of the measuring electrode are placed in the hollow part of the key, and the auxiliary electrode is immersed in a glass with electrolyte. A serious disadvantage of using the described constructions for the determination of fluorides is the presence of a contact between the analyzed solution and glass surface of electrolytic key, which makes it impossible to determine the low contents of the fluoride ion because of its interaction with the glass. In addition, it should be noted a sufficiently long time, necessary for complete wetting of the asbestos filament of the analyzed solution and, as a consequence, the establishment of constant millivoltmeter readings (according to the authors' observations of 15-20 minutes).
The goal of our investigation was to develop a robust, simple and convenient method for the determination of fluoride ion in 0.05-0.5 cm3 sample volumes which would be suitable for batch analysis and would not require complex instrumentation. Of particular interest was the development of a method for analyzing the fluoride ion in biological samples that have small volumes and low concentrations of the analyte.
MATERIALS AND METHODS
Fluoride electrode (ELIT-221) and silver chloride reference electrode (EVL-1М3) were used in the current study. The electrode potential was measured with ± 0.1 mV accuracy on a HANNA-221 pH-meter/millivoltmeter at ambient temperature (22-24°С).
All chemicals and reagents were purchased from Reachim (Moscow, Russia) and used as such without any further purification. The following buffer solution (g/dm3): NaCl – 58.50; trisodium citrate pentahydrate Na3C6H5O7 ∙ 5Н2О – 0.36; CH3COONa∙3Н2О – 102.00; CH3COOH – 14.40 was added to the sample in a 1:1 ratio (V/V) [8]. Stock solution of sodium fluoride with the concentration of fluoride ion 2 mg/cm3 was prepared by dissolving of 2.210 g NaF (ultra high purity) in bidistilled water in a 0.5 dm3 volumetric flask. Solutions with lower concentrations were prepared by diluting the stock solution with bidistilled water.
Between sample measurements, the electrodes were washed with bidistillated water and dried with a filter paper. The calibration curves were constructed using the data for 0.2, 0.4, 0.6, 0.8, 1.0 and 2.0 mg/dm3 fluoride ion concentrations.
RESULTS DISCUSSION
We suggest to perform potentiometric measurements on an apparatus presented in Fig.1 using standard 1.5 cm3 polystyrene sample cups connected by a salt bridge.
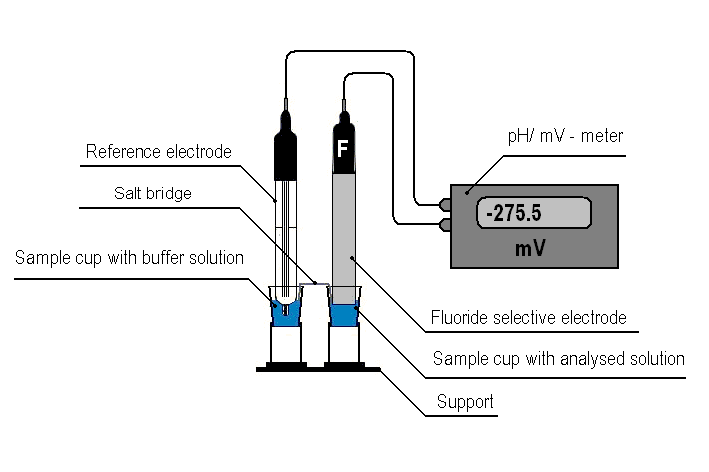
Figure 1. Apparatus for the potentiometric microdetermination of fluoride ion.
The main electrode is introduced to a sample cup filled with the sample diluted with the buffer solution while the reference electrode is introduced to a sample cup with the buffer solution diluted with bidistilled water with a 1:1 ratio (V/V). Sample cups are connected by a salt bridge which is strip a porous material (e.g. filter paper), wetted buffer solution, diluted with water in a ratio of 1: 1 (V/V). Supports for the vials are made from 15 cm3 plastic test-tubes with their lower parts cut, and are fastened to a plastic base with screws.
To evaluate the correctness of the suggested method we compared the calibration curves (E dependence to inverse negative logarithm of the concentration of the fluoride ion in the standard solution (mg/dm3) ) (Fig. 2) and the measurement results for water from various sources (Table 1) obtained by a standard method [8] using 20 cm3 sample volumes and by the micromethod using 0.5 cm3 sample volumes
Table 1. Fluoride ion content in water, mg/dm3 (n = 3; P = 0.95)
|
Sample |
Standard method |
Micromethod |
|
Spring water |
0.21 ± 0.02 |
0.24 ± 0.02 |
|
Tap water 1 |
0.59 ± 0.03 |
0.58 ± 0.03 |
|
Tap water 2 |
1.58 ± 0.15 |
1.65 ± 0.16 |
|
Wellbore water 1 |
1.74 ± 0.10 |
1.73 ± 0.10 |
|
Well water 2 |
1.67 ± 0.16 |
1.66 ± 0.16 |
The data in Fig. 2 and Table 1 demonstrate that the experimental calibration curves and the analysis results are identical within the bounds of expiremental error. The suggested micromethod allows the analysis of sample volumes as low as 0.050 cm3. Its important advantage is economic efficiency: at least 10-fold reduction in the number of necessary reagents is achieved due to the use of small volumes of samples, with the same rapidity of analysis (about 3-4 minutes).
The developed micromethod was tested in the determination of the fluoride ion in biological objects by the example of an extract of water-soluble proteins of the hepatopancreas of the mollusk Viviparus viviparus L. when studying the accumulation of fluorine in model conditions.
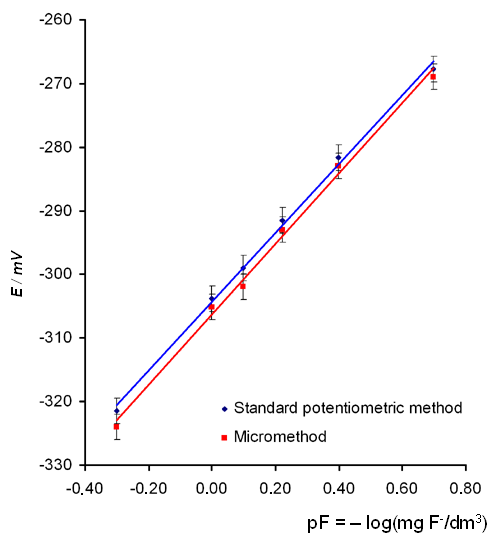
Figure 2.Calibration curve for fluoride determination. The equation of best-fit line is
Standard potentiometric method: E = 53.9 pF – 304.2, R2 = 0.9976
Micromethod: E = 50.9pF – 306.6, R2 = 0,9925
The volume of the extract, according to [9], is 0,1-0,2 cm3, which makes it impossible to use standard potentiometric techniques for its analysis. Sample preparation to eliminate the effect of organic components performed it according to the following procedure: 0.1 cm3 of the extract was placed in a glassy carbon crucible, 0.1 cm3 of 50% hydrogen peroxide solution and 0.1 M sodium hydroxide solution was added and the solution was evaporated to dryness when heated on a electric oven. The operation was repeated 5 times. The resulting precipitate was dissolved in 0.5 cm3 of distilled water and quantitatively transferred to a polypropylene tube and was added of a 0.5 cm3 total ionic strength adjustment buffer, then the electrodes were immersed in the solution to be analyzed and the EMF value was measured. The concentration of fluoride ion was determined from the calibration graph. The results of determination of fluorine in the hepatopancreas extracts of mollusks, which were in water with an increased and background content of fluoride ion, are given in Table. 2.
Table 2. Fluoride ion content in hepatopancreas extracts of mollusks, mg/dm3 (n = 3; P = 0.95)
|
Образец |
Fluoride ion content |
|
1 |
2.4 ± 0.2 |
|
2 |
3.3 ± 0.4 |
|
3 |
6.3 ± 0.5 |
|
4 |
11.0 ± 0.5 |
From the data in Table 2, it can be seen that the content of fluorine in extracts of hepatopancreas of mollusks that were in water with a high content of fluorine (samples 3, 4) significantly exceeds its content in the hepatopancreas of mollusks in the water with its background content (samples 1,2).
It should be noted that the application field of the developed method is not limited by the determination of fluoride, an analogous apparatus can be employed for potentiometric determinaion of other ions. The current method can be conveniently used for the potentiometric determination of chloride and nitrate ions as it allows common silver chloride reference electrodes to be employed instead of the more complex and expensive double salt bridge electrodes while eliminating the risk of contaminaton of the solutions under study with potassium chloride from the reference electrode which would hinder the analysis.
CONCLUSION
A simple method for the determination of fluoride ion in sample volumes of 0.05-0.5 cm3 was suggested and appraised for chemical analysis of natural objects on an example of an extract of a hepatopancreas of a freshwater mollusc Viviparus viviparus L. The method is convinient for batch analysis and does not reqiure the construction of a complex apparatus.
REFERENCES
- Midgley, D.; Torrance, K. Potentiometric Water Analysis, I ed.; Mir: Moscow, 1980; pp 404 – 415.
- Наrа, Н.; Yabuuchi, К.; Higashida, M.; Ogawa, M. Determination of free and total fluoride in rain water using a continuous-flow system equipped with a fluoride ion-selective electrode detector. Anal. Chim. Acta. 1998, 364, 117 – 123.
- Santos, J.R.; Lapa, R.A.; Lima, J.L. Development of a tubular fluoride potentiometric detector for flow analysis: Evaluation and analytical applications. Anal. Chim. Acta. 2007, 583, 429 – 436.
- Brambilla, E.; Felloni, A.; Fadini, B.L.; Strohmenger, C.L. A simplified micromethod for fluoride analysis. Arch. Oral Biol. 1998, 43, 819 – 823.
- Durst, R.A.; Taylor, K. J. Modification of the fluoride activity electrode for microchemical analysis. Anal. Chem.. 1967, 39, 1483 – 1485.
- Tyler, J.E.; Poole, D.F.G. The rapid measurement of fluoride concentrations in stored human saliva by means of a differential electrode cell. Arch. Oral Biol. 1989, 34, 995 – 998.
- Rachinsky F.Yu., Rachinskaya M.F. Technique of laboratory works. L.: Chemistry, 1982. - 432 p.
- Guidance document No 52.24.360-95. Mass concentration of fluoride in the water. Measurement technique of potentiometric method with the ion-selective electrode: Rostov-on-Don, 2008; 11 p.
- Droganovа, Т. S.; Polykarpova, L.V.; Petrenko, D.B.; Vasiliev N.V. Acid phosphatase activity in the hepar of a river snail (Viviparus viviparus L.) under the influence of fluoride ion. Bulletin of Moscow State Regional University. Series Natural Sciences. 2014 (No 5), 14 – 19.
Recommended for publication by Prof. S.N. Osipov
Fluorine Notes, 2018, 118, 1-2
