Received: February 2018
DOI 10.17677/fn20714807.2018.02.03
Fluorine Notes, 2018, 117, 5-6
Synthesis and properties of the new monomer - 1,1'-di[methacryloyloxy -bis(trifluoromethyl)methyl]ferrocene
V.I. Dyachenko, O.A. Mel'nik, L.N. Nikitin, S.M. Igumnov
A.N. Nesmeyanov Institute of Organoelement Compounds Russian Academy of Sciences (INEOS RAS), Russian
Federation, 119991, GSP-1, Moscow, V-334, Vavilova St. 28
e-mail: vic-d.60@mail.ru
Abstract:1,1'-di[methacryloyloxy-bis(trifluoromethyl)methyl]ferrocene was synthesized by the reaction between 1,1'-di(1-hydroxy-1-trifluoromethyl-2,2,2-trifluoroethyl)ferrocene and methacryloyl chloride in the presence of sodium hydride in anhydrous dimethylformamide (DMF). Using the physicochemical methods, the structure of 1,1'-di[methacryloyloxy-bis(trifluoromethyl)methyl]ferrocene and it’s ability to radical copolymerization with methylacrylate with cross-linked copolymers formation were established.
Keywords: 1,1'-di(1-hydroxy-1-trifluoromethyl-2,2,2-trifluoroethyl)ferrocene, copolymerization, 1,1'-di[methacryloyloxy-bis(trifluoromethyl)methyl]ferrocene, hydrophobic coatings
The fluorinated polymers, in contrast to its non-fluorinated analogues, are more hydrophobic and oleophobic, highly resistant to oxidation, action of acids and other aggressive media. As a result of these properties, its expansion, as the functional hydrophobic coatings, in the science of materials, aircraft and mechanic engineering, textile industry, everyday usage is increasingly growing up. [1]. Today, the investigations in the sphere of chemistry of coordination polymers are also in intensive progress. Among them, the elementoorganic polymers, which have ferrocenyl substituent in its composition, take a significant place. [2,3]. Ferrocene-containing polymers are mainstream for glucosic biosensors development [4], for producing of organic polyelectrolytes [5], liquid crystalline polymers [6], compound materials [7]. Earlier we reported the synthesis of 1-trifluoromethyl-1-ferrocenyl-2,2,2-trifluoroethylmethacrylate [8] and preparation of «side-chain» (co)polymers of different composition based on this compound [9]. It was shown that it’s addition (3-5%) as a comonomer to the reaction mixture while methylmethacrylate polymerization leads to substantial increase in the thermal destruction temperature of the forming polymethylmethacrylate [10].
The aim of this research is a preparation of a new fluorine-containing monomer (Scheme 1) having two methacrylate groups in ferrocene’s cyclopentadienyl rings, capable of copolymerization with vinyl monomers to form cross-linked polymers. Furthermore, due to the ferrocene centre in its molecule, screened by two volume tertiary substituents, it is also able to act as an antioxidant, thus extending the temperature range of the application of it's copolymers.
Thus, by the reaction of 1,1'-di(1-hydroxy-1-trifluoromethyl-2,2,2-trifluoroethyl)ferrocene (1) with excess of methacryloyl chloride (2) corresponding 1,1'- di[methacryloyloxy-bis(trifluoromethyl)methyl]ferrocene was obtained (3).
Scheme 1
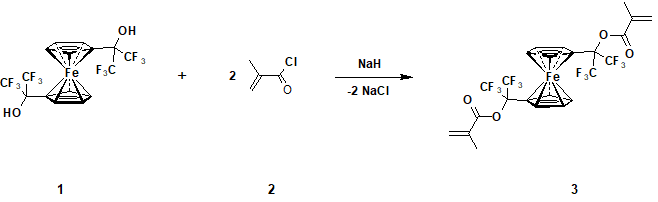
The reactions between 1 and 2 can be easily carried out in anhydrous DMF in the presence of sodium hydride with the formation of 3 with the yield 77% (Scheme 1). The initial, for this synthesis, dicarbinol 2 was obtained by the reaction of ferrocene with hexafluoroacetone catalyzed by trifluoroacetic acid with the yield 75%, as it was described earlier [11]. It should be noted that carbinol 2 can be obtained without the use of catalyst by prolonged heating of these reagents (180°С, 96 h) [12]. Raw materials for the synthesis of the compound 3 (ferrocene, hexafluoroacetone and methacryleoylchloride) are produced in commercial scale, which makes it quite affordable.
Dimethacrylate 3 is a red-orange crystalline compound, non-soluble in water and soluble in almost all organic solvents, stable in storage. It’s structure was proved by1H NMR and19F spectra, IR-spectroscopy and elemental analysis data. In the IR-spectra 3 we observe the fringes of absorption, corresponding to ferrocene’s centre fragments (zone of valence stretching of CH-vibrations in the field of 3148 cm-1, zone of nonplanar deformation vibration of CH-bonds of substituted Сp-rings in the field of 854 см-1, zones in the field of 1023−1079 см-1 which are typical for ferrocene’s homoanular derivatives and zone of double degenerated antisymmetric streching vibration Fe-Cp in the field of 493 cm-1). Besides that, in spectra of compound3there are zones of stretching vibrations С=С at 1634 cm-1 and deformation vibrations С=С at 969, 984 cm-1. We also can observe zones of absorption of С=О and С−О bonds in the field of 1755 и 1135 сm-1correspondingly. Zones at 1224, 1204 cm-1, that are typical for СF3-groups and zones in the field 2967 cm-1, that are typical for СН3- groups also take place.
Due to the presence of two groups with multiple bond in its molecule, dimetacrylate 3 is able to copolymerize with methylmethylacrylate with cross-linked polymers formation in the presence of an initiator of the radical polymerization - azoisobutyronitrile at 60-70°С, similar to 1-trifluoromethyl-1-ferrocenyl-2,2,2-trifluoromethylacrylate [8] ( Pic.1).
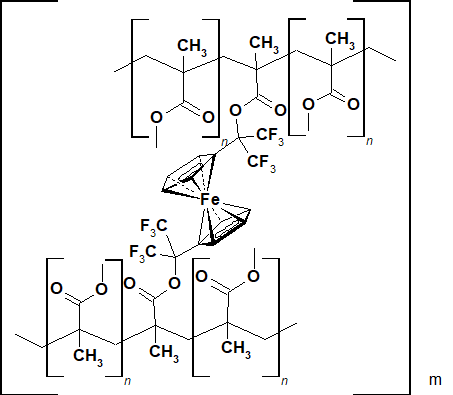
Pic.1
It was found that an addition of this compound (1%) in the reaction mixture raises the temperature of the thermal destruction (Td) of the resulting polymethymethacrylate by 50-55°С in the air.
Now it is being studying the possibility of usage of 3 in order to obtain redispercible copolymers [13,14] for raising the contact angle of wetting θ of the hydrophobic coatings formed [15].
Experimental
1H, 19F NMR spectra were recorded on the instrument «Bruker Avance 400» with the operational frequencies 400,13 MHz, 376,5 MHz correspondingly. Сhemical shifts of 1Н were recorded using tetramethylsilane (TMS) as an internal standard, 19F with CF3CO2H as an external standard.
IR-spectra were recorded on Nicolet Magna-750. Mass-spectra were recorded on Finnigan MAT INCOS 50 (direct input, ionization energy 70 eV).
1,1'-di[methacryloyloxy-bis(trifluoromethyl)methyl]ferrocene (3). To 1,04 g (2 mmol) of carbinol 2 in 5 ml of anhydrous dimethylformamide at the temperature 20°С while mixing 0,08 g (2,5 mmol) of sodium hydride 60% in paraffinic oil was added by portions. When the release of hydrogen is finished, 5 mg of ionol was added to the solution and then 0,29 g (2,5 mmol) of methacryloylchloride was added dropwise at 20°С. The reaction mixture was mixed during 2 h and then poured into 20 ml of cold water. The product of the reaction was extracted by petroleum ether (2х20 ml), extract was dried with anhydrous sodium sulfate, and evaporated in vacuum. The compound 3 was purified by column chromatography, eluent - hexane, 0.9 g of red-orange oil crystallized upon cooling was obtained. Yield 77%. Rf=0,55 (СHCI3), Melting point 45-46°С (petroleum ether).
1Н NMR Spectra δ, ppm: 6,19 (w.s. с, 1Н,=CН2); 5,73 (w.s. с, 1Н,=CН2); 4,49 (br.s., 8Н, 2С5Н4); 1,95 (br.s., 6Н, 2СН3). Spectra NMR 19F, δ, ppm.: 6,28 (s). Mass-spectrum, m/z (Irel, %): 654 [M]+ (100), 586 (34), 570 (10), 226 (27), 195 (19), 69 (7). Found, %: С 44,33; Н 2,71; F 34,78. С24H18F12FeO4. Calculated, %: C 44,06; Н 2,77; F 34,85. Intensive molecular ion 654 [M]+(100) of the compound 3 indicates it’s stability, owing to screenage by СF3-groups, and also abilities to enter the redox processes because of its reversible valence change (Fe2+)/(Fe3+), without the molecule geometry change.
Conclusions
The new monomer − 1,1'-di[methacryloyloxy-bis(trifluoromethyl)methyl]ferrocene was synthesized and it’s physicochemical properties were studied. On the methylmethacrylate, taken as an example, there was shown it’s ability to copolymerization with vinyl monomers, which results in cross-linked copolymers formation. It’s addition (1%) to the reaction mixture, raises the thermal destruction temperature of forming polymethymethacrylate (Тd), on 50-55°С in the air.
Now it is being studying the possibility of use of 3 in order to obtain redispercible copolymers for rising the contact angle of wetting θ of the formed hydrophobic coatings.
Acknowledgements
The authors are thankful for recording 1Н, 19F NMR, IR- and mass-spectra and for elemental analysis to A.N. Nesmeyanov Institute of Organoelement Compounds Russian Academy of Sciences (INEOS RAS).
This work was financially supported by the Russian Foundation for Basic Research (grant 16-29-05334-ofi-m)
References
- Fluorine compounds. Synthesis and Application, ed. N. Ishikawa, translation from Jap., Mir, 1990.
- D. Wohrle, A. Pomogailo. Metal complexes and metals in macromolecules: synthesis, structure and properties. Weinheim: Wiley, 2003, 667 p.
- W.A. Amer, L. Wang, A.M. Amin, L. Ma, H. Yu. J. Inorg. Organomet. Polym. Mater., 2010, 20, 605-615.
- (a) J. Gun, O. Lev, Anal. Chim. Acta., 1996, 336, 95-106; (b) J. Gun, O. Lev, Anal. Lett. 1996, 29, 1933-1938].
- 5. Ye Gao and Jean'ne M. Shreeve, Journal of Polymer Science Part A: Polymer Chemistry, 2005, 43, 5(1), 974–983.
- S. Senthil, P. Kannan, Journal of Polymer Science Part A: Polymer Chemistry, 2001, 39, 14, 2396-2403.
- S.M. Igumnov, V.I. Dyachenko, O.A. Mel’nik, V.I. Sokolov, L.N. Nikitin // Chapter 11 in the book «Fluoropolymer materials», ed. V.M.Buznik, Publishing House of NTL, Tomsk, 634050, pl. Novosobornaya, 1, ISBN 978-5-89503-596-2, 2017, p.472-503
- V.I. Dyachenko, L.N. Nikitin, O.A. Mel'nik, S.M. Peregudova, A.S. Peregudov, S.M. Igumnov, A.R. Khokhlov. Fluorine notes, 2011, 79(6).
- O.A. Mel’nik, V.I. Dyachenko, L.N. Nikitin, I.V. Blagodatskikh, M.I. Buzin, S.M. Peregudova, Ya.S. Vygodskii, A.R. Khokhlov. Doklady Chemistry, 2012, 443 (2), 107-110.
- O.A. Mel’nik, V.I. Dyachenko, L.N. Nikitin, I.V. Blagodatskikh, M.I. Buzin, G.Yu. Yurkov, Ya.S. Vygodskii, S.M. Igumnov, V.M. Buznik. Polymer Science, Ser.A, 2013, 55 (11), 625-630.
- V.I. Dyachenko, A.F. Kolomiets, A.V. Fokin, Abstracts of the Fifth All-Union Conference on Organometallic Chemistry, Riga, 1991.
- V. Albrow, A.J. Blake, A. Chapron, C. Wilson, S. Woodwar. Inorg. Chim. Acta, 2006, 359, 1731–1742.
- S.Yu. Tuzova, A.Yu. Nikolaev, L.N. Nikitin, A.A. Pestrikova, I.Y. Gorbunova //Journal of Inorganic Chemistry, 2015, 60 (6), 800-805.
- S.Yu. Tuzova, A.A. Pestrikova, L.N. Nikitin, A.Yu. Nikolaev, I.Yu. Gorbunova, E.M. Antipov, M.M. Kuzmina. Patent RU 2610512, 2017.
- S.Yu. Tuzova, A.A. Pestrikova, L.N. Nikitin, A.Yu. Nikolaev, Patent RU 2618253, 2017.
Recommended for publication by Prof. Sergei R. Sterlin
Fluorine Notes, 2018, 117, 5-6
