Received: December 2017
DOI 10.17677/fn20714807.2018.01.02
Fluorine Notes, 2018, 116, 3-4
Synthesis of Polychlorofluoroarenes from Polyfluoroarenethiols, SOCl2 and SO2Cl2
P. V. Nikul’shin, А. M. Maksimov, R.A.Bredikhin, and V. Е. Platonov
N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry, Siberian Branch of the Russian Academy of Sciences, pr. Academika Lavrent’eva 9, Novosibirsk, 630090, Russian Federation
e-mail: platonov@nioch.nsc.ru
Abstract. The thiol group in polyfluoroarenethiols was replaced by the chlorine atom, using SOCl2 and SO2Cl2 as chlorinating reagents. By heating in ampules at 200–220°C polyfluoro- and polychlorofluoroarenethiols with SO2Cl2 monochloro- and dichloropolyfluoroarenes and also 1,2,4-trichlorotrifluorobenzene were synthesized. Dichloropolyfluoroarenes contain chlorine atoms in ortho- and para-positions.
Keywords: Thionyl chloride, sulfuryl chloride, thiol group, polyfluoroarenethiols, polychlorofluoroarenes
Polychlorofluoroarenes are important products for the synthesis of a large number of polyfluoroaromatic compounds with various functional groups. Selective participation of the CAr-Cl bond in the metallation reactions is one of the directions of transformation of chloropolyfluoroarenes. The Grignard reagents [1], polyfluoroaryl lithiums [1] and polyfluoroarylzinc compounds [2] formed from chloropolyfluoroarenes are versatile and convenient to use for the synthesis of a large number of functional derivatives of polyfluoroaromatic compounds. In this connection, in recent years, we have been developing a method of synthesis of chloropolyfluoroarenes consisting in substitution of the thiol group in polyfluoroarenethiols by chlorine atom. By this method a series of chloropolyfluoroaromatic compounds was synthesized in high yields [3, 4]. The process was realized at high temperature (~400°C) in a flow reactor as join pyrolysis of polyfluoroarenethiols with Cl2 as well as with SOCl2 and SO2Cl2 as chlorine sources [3]. In addition to the reactions in the flow system, we studied the conversion of polyfluoroarenethiols with PCl5 in ampoules at ~ 200°C, which also led to the preparation of chloropolyfluoroarenes [4]. On the example of the reaction of pentafluorobenzenethiol 1 with Cl2 and PCl5, carried out under different conditions, the scheme for the replacement of the thiol group by the chlorine atom was suggested. This scheme includes the intermediate formation of pentafluorobenzenesulfenyl chloride 2 and its conversion under the action of Cl2 and PCl5 to chloropentafluorobenzene 3 with the participation of an intermediate radical σ- complex [3, 4].
Use in the gas phase process, along with Cl2, thionyl chloride and sulfuryl chloride as chlorine sources, characterizes the reaction of substitution of the thiol group in polyfluoroarenethiols by the chlorine atom in the methodical and practical sense as a rather general way of synthesis of chloropolyfluoroarenes. The reactions of polyfluoroarenethiols with PCl5, carried out in ampoules at a lower temperature (~200°C) [4], are additional evidence in favor of such conclusion. We have also studied the conversion of polyfluoroarenethiols with SOCl2 and SO2Cl2 in ampoules under similar conditions to increase the possibilities of this type of reactions.
We have shown that when thiol 1 was heated with SOCl2 or SO2Cl2 in ampoules at ~200°C, arene 3 was obtained with high yields. It was found that the reaction of thiol 1 with SOCl2 requires a longer time to achieve a good yield of arene 3 than in the case of using SO2Cl2 at the same temperature of process (Scheme 1).
Scheme 1
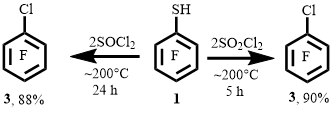
When the reaction time of thiol 1 with SOCl2 was reduced from 24 h to 5 h at ~200°C, the reaction mixture contained arene 3, the supposed decafluorodiphenylpolysulfanes and sulfenyl chloride 2 in a ratio of ~ 55: 34: 11, respectively (according to NMR 19F).
In the reaction of thiol 1 with SO2Cl2 at ~200 ° C for 2.5 h, the reaction mixture contained arene 3 in a larger amount than in the case of the reaction of thiol 1 with SOCl2 for 5 h. In addition to arene 3, the supposed decafluorodiphenylpolysulfanes and sulfenyl chloride 2 were also present in the mixture. The ratio of these products was ~ 79: 18: 3, respectively (according to NMR 19F). However, a comparison of the results of the reactions of thiol 1 with SOCl2 (5 h) and SO2Cl2 (2.5 h) is relative, since the mass yields of the reaction mixtures have not been evaluated.
The above results of the reactions of thiol 1 with SOCl2 and SO2Cl2 may indicate that the process involving SO2Cl2 takes less time than using SOCl2. Therefore, we further carried out the reactions of polyfluoroarenethiols with SO2Cl2.
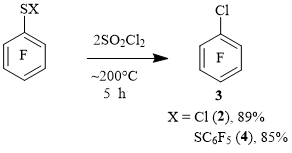
Sulfenyl chloride 2 and decafluorodiphenylpolysulfanes formed in the reaction of thiol 1 with SO2Cl2 at ~ 200°C for 2.5 hours can be of interest to obtain arene 3 from them under the action of SO2Cl2. In this connection, the reaction of sulfenyl chloride 2 with SO2Cl2 (~ 200 ° C, 5 h) was carried out. As a representative of these polysulfanes, it seemed reasonable to carry out the reaction of decafluorodiphenyldisulfane 4 with SO2Cl2 in the reaction conditions of thiol 1 with SO2Cl2 (~200°C, 5 h).
As it turned out, when sulfenyl chloride 2 and disulfane 4 were heated with SO2Cl2, compound 3 was also obtained in high yields (Scheme 2).
Scheme 2
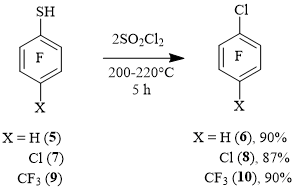
A similar process occurs in the case of para-substituted derivatives of thiol 1. Thus, when 2,3,5,6-tetrafluorobenzenethiol 5 was heated with SO2Cl2, 1-chloro-2,3,5,6-tetrafluorobenzene 6 was obtained. The reaction of 4-chloro-2,3,5,6-tetrafluorobenzenethiol 7 with SO2Cl2 gave 1,4-dichloro-2,3,5,6-tetrafluorobenzene 8. When 4-tifluoromethyl-2,3,5,6-tetrafluorobenzenethiol 9 was reacted with SO2Cl2, 4-chloroheptafluorotoluene 10 was obtained in a high yield (Scheme 3).
Scheme 3
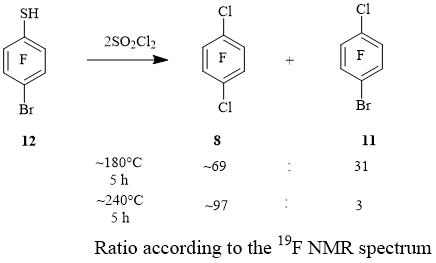
At the same time, the attempt to obtain 1-bromo-4-chloro-2,3,5,6-tetrafluorobenzene 11 by chlorination of 4-bromo-2,3,5,6-tetrafluorobenzenethiol 12 at ~ 180°C showed that in this reaction the replacement of the bromine atom by chlorine occurs also to a large extent. With an increase in the reaction temperature to ~ 240°C, compound 8 was obtained with a small admixture of arene 11 (Scheme 4). We have previously shown that a similar process occurred in the reaction of thiol 12 with PCl5 under similar conditions [4].
Scheme 4
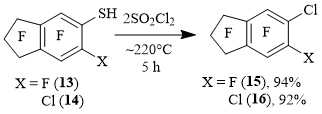
5-Chlorononafluoroindan 15 and 5,6-dichlorooctafluoroindan 16 were obtained from 5-nonafluorindanthiol 13 and 6-chlorooctafluoroindan-5-thiol 14, respectively (Scheme 5).
Scheme 5
Heating of a mixture of dichlorotrifluorobenzenethiols with SO2Cl2 in an ampoule was used to synthesize practically individual 1,2,4-trichlorotrifluorobenzene 17 (Scheme 6)..
Scheme 6
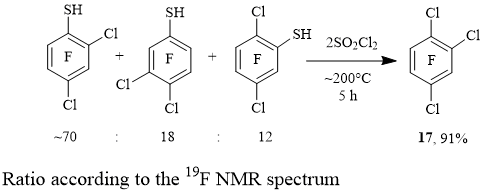
The isomeric mixture of dichlorotrifluorobenzenethiols was prepared from a technical mixture of o-,
m-, p-C6Cl2F4 and KSH (90% yield) [5].
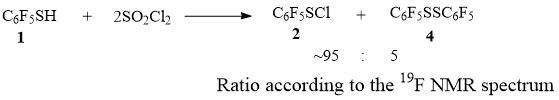
When
thiol 1 is mixed with SO2Cl2 at room temperature, mainly sulfenyl
chloride 2 is formed, along with a small amount of disulfane 4 (according to NMR 19F, Scheme 7).
Scheme 7
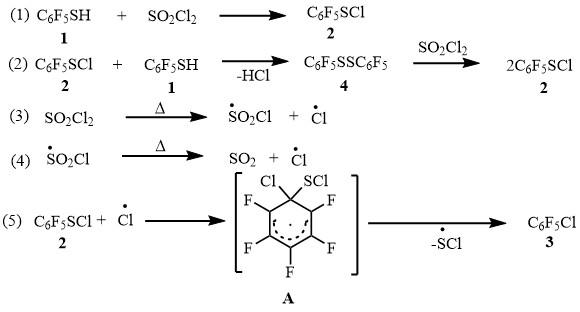
It is possible that compound 4 in the reaction with SO2Cl2 is converted to sulfenyl chloride 2 [6]. The latter, under the action of the chlorine atom formed from SO2Cl2 [7-9] (Equations 3 and 4, Scheme 8), is converted to arene 3, including the intermediate formation of the radical σ-complex A [4]. Probably, the reaction of thiol 1 with SO2Cl2 in the flow system at ~ 400°C, described by us earlier [3], proceeds in a similar manner.
Scheme 8
EXPERIMENTAL
Authors would like to acknowledge the Multi-Access Chemical Service Center SB RAS for spectral and analytical measurements.
The 19F and 1H NMR spectra were recorded on a Bruker AV-300 [282.4 (19F) and 300 (1H) МHz] spectrometer in CCl4 with added (CD3)2СO, internal reference С6F6 and HMDS (0.04 ppm from TMS). Positive values of chemical shifts correspond to the signal downfield shift.
GC analysis was carried out on gas chromatograph LKhM-72 with a detector of thermal conductivity (TDC) and packed (stuffed) columns 2 m long and 4 mm inside diameter, which were filled with a solid inert carrier of Chromosorb W-AW-DMCS, impregnated with a liquid stationary phase (dimethyl polysiloxane BC-1 or dimethyltrifluoropropyl polysiloxane SCTF-50) in an amount of 15% of the mass of the carrier. The flow rate of helium through each of the columns is 60 ml/min. The temperature of the evaporator is 280ºC, the initial temperature of the column is 50ºC -1 min, 10 deg/min to 280ºC, the isotherm at 280ºC before the exit of all components of the sample, the temperature of TDC 280ºC.
Initial polyfluoroarenethiols were obtained by the method [10].
The formation of compounds 2 and 4 and the final products by reactions of the polyfluoroarenethenthiols chlorination was confirmed by comparing the chemical shifts and spin-spin coupling constants of the 19F and 1H NMR spectra of these compounds with the literature data [3, 5, 11].
Method 1. Polyfluoroarenethiol was placed in an ampoule and SOCl2 or SO2Cl2 was added by portions. After the end of the visible gas evolution, the ampoule was sealed, placed into a metal case, and heated. After the reaction completed, the ampoule was cooled, opened, its content was placed into a flask under a layer of water with ice (80–100 g), and subjected to steam distillation. The reaction product was separated, dried with CaCl2, and analyzed by GC, 19F and 1H NMR methods.
Similarly, the reactions of sulfenyl chloride 2 and disulfane 4 with SO2Cl2 were carried out. In this case, the ampoule was sealed immediately after the reagents were placed in it.
Reactions of pentafluorobenzenethiol 1 with SOCl2. From 2.77 g (13.84 mmol) of compound 1 and 3.30 g (27.74 mmol) of SOCl2 (200–202°C, 24 h), 2.52 g of compound 3 were obtained (GC content: 97.3%), yield: 88%.
The results of the reactions of polyfluoroarenethioles (1, 5, 7, 9, 13, 14, a mixture of C6Cl2F3SH, and also compounds 2 and 4) with SO2Cl2 are given in Table 1.
Table 1. Synthesis of chloropolyfluoroarenes
|
Run No. |
Substrate, g (mmol) |
SO2Cl2, g (mmol) |
The molar ratio of the substrate to SO2Cl2 |
Temp. (ºC) |
Time (h) |
Yield of mixture (g) |
The content (yield) of the product by GC (%) |
|
1 |
1, 2.76 (13.79) |
4.01 (29.95) |
2.17 |
203-205 |
5 |
2.55 |
3, 98.3 (90) |
|
2 |
2, 3.01 (12.83) |
3.76 (21.21) |
2.19 |
200-202 |
5 |
2.37 |
3, 98.0 (89) |
|
3 |
4, 3.05 (7.66) |
2.08 (15.53) |
2.03 |
203-205 |
5 |
2.67 |
3, 91.2 (85) |
|
4 |
5, 2.24 (12.30) |
3.40 (25.39) |
2.06 |
200-202 |
5 |
2.07 |
6, 99.0 (90) |
|
5 |
7, 3.29 (15.19) |
4.21 (31.41) |
2.07 |
203-205 |
5 |
2.91 |
8, 99.1 (87) |
|
6 |
9, 2.39 (9.55) |
2.88 (21.51) |
2.25 |
218-220 |
5 |
2.17 |
10, ~100 (90) |
|
7 |
13, 1.98 (6.34) |
1.79 (13.37) |
2.11 |
220-222 |
5 |
1.89 |
15, 99.4 (94) |
|
8 |
14, 3.00 (9.13) |
2.54 (18.97) |
2.08 |
218-220 |
5 |
2.80 |
16, 99.3 (92) |
|
9 |
a mixture of C6Cl2F3SH, 3.04 (13.37) |
3.87 (28.90) |
2.22 |
203-205 |
5 |
2.82 |
17, 99.4 (91) |
0.15 g (0.75 mmol) of compound 1 was mixed in a flask with 0.21 g (1.57 mmol) of SO2Cl2 at room temperature. At the end of the gas evolution, the resulting mixture was compounds 2 and 4 in a ratio of ~ 95: 5, respectively (19F NMR data).
Method 2. Compound l was placed in an ampoule and SOCl2 or SO2Cl2 was added by portions. After the end of the visible gas evolution, the ampoule was sealed, placed into a metal case, and heated. After the reaction completed, the ampoule was cooled, opened, its content was dissolved in ~ 2 ml of methylene chloride and analyzed by 19F NMR.
Heating of 0.12 g (0.60 mmol) of compound 1 with 0.16 g (1.35 mmol) of SOCl2 at 202-204°C for 5 h gave a mixture of compounds 2, 3, 4 (NMR 19F) and also probably decafluorodiphenyltrisulfane (δ, ppm: 13.5 (F-4 and F- 4') [12]) and decafluorodiphenyltetrasulfane (δ, ppm: 13.4 (F-4 and F-4') ( [12]) contained in the methylene chloride solution. In these two polysulfanes, chemical shifts of meta-atoms of fluorine are in the region of 2.1÷2.6 ppm [12], ortho- atoms of fluorine are in the region of 31÷32 ppm [12]. The ratio of compounds 2, 3, 4 and a mixture of decafluorodiphenyltri- and -tetrasulfane was ~ 11: 55: 10: 24, respectively (19F NMR data).
Similarly, heating of 0.20 g (1.00 mmol) of thiol 1 with 0.27 g (2.02 mmol) of SO2Cl2 at 200-202°C for 2.5 h gave compounds 2, 3, 4 and a mixture of decafluorodiphenyltri- and -tetrasulfane in a ratio of ~ 3: 79: 3: 15, respectively (19F NMR data).
Reactions of 4-bromo-2,3,5,6-tetrafluorobenzenethiol (12) with SO2Cl2. From 0.32 g (1.23 mmol) of compound 12 and 0.34 g (2.54 mmol) of SO2Cl2 (180-182°C, 5 h), a mixture containing compounds 8 and 11 in a ratio of 69: 31, respectively,) was obtained (NMR 19F data). When the reaction temperature was increased to 238-240°C (5 h), heating of 0.27 g (1.03 mmol) of compound 12 and 0.30 g (2.24 mmol) of SO2Cl2 gave a mixture containing compounds 8 and 11 in a ratio of 97: 3, respectively (19F NMR data).
The work was supported by the Russian Foundation for Basic Research (Project No. 15-03-08869a).
References
- Chambers R.D. Fluorine in Organic Chemistry. Wiley, New York, 1973.
- Vinogradov A.S., Krasnov V.I., Platonov V.E., Organozinc reagents from polyfluoroarenes: Preparation and reactions with allyl halides. Synthesis of allylpolyfluoroarenes. Russ J. Org. Chem. 2008. V. 44. № 1. P. 95-102.
- Platonov V.E., Maksimov A.M., Dvornikova K.V., Nikul’shin P.V. Organofluorine sulfur-containing compounds: V. Joint pyrolysis with chlorine or bromine of polyfluoroarenethioles, polyfluorohetarenethioles, and their derivatives. Russ J. Org. Chem. 2005. V. 41. № 11. P. 1647-1653.
- Nikul’shin P.V., Maksimov A.M., Platonov V.E. Synthesis of Chloropolyfluoroarenes from Polyfluoroarenethiols and PCl5. Russ J. Org. Chem. 2016. V. 52. № 2. P. 200-205.
- Nikul’shin P.V., Maksimov A.M., Platonov V.E. Synthesis of 1,2-dichlorotetrafluoro- and 1,2,4-trichlorotrifluorobenzenes. Russ J. Org. Chem. 2012. V. 48. № 4. P. 536-543.
- Still I.W.J., Kutney G.W., McLean D. Convenient Method for the Conversion of Thiols and Disulfides to the Corresponding Chlorides. J. Org. Chem. 1982. V. 47. № 1. P. 560-561.
- Nonhebel D.C., Walton J.C. Free-Radical Chemistry. Structure and Mechanism. Cambridge: At the University Press, 1974.
- Poutsma M.L. Methods in Free Radical Chemistry. New York: Marcel Dekker, 1969. V. 1. P. 111-115.
- Mathieu J., Panico R. Mecanismes Reactionnels en Chimie Organique. Paris: Hermann, 1972.
- Maksimov A.M., Platonov V.E. Reactions of some polyfluoroaromatic compounds with potassium hydrosulfide. Fluorine Notes.1999. Vol. 4. http://notes.fluorine1.ru/contents/history/1999/4_1999/letters/index.html.
- Nikul’shin P.V., Maksimov A.M., Platonov V.E.Synthesis of chloro- and ortho-dichloropolyfluoroarenes by pyrolysis of polyfluoroarenes in the presence of chlorine. Rus. J. Appl. Chem. 2010. V. 83. № 7. P. 1254-1258.
- Peach M. E. Pentafluorophenyl polysulfides. Intern. J. Sulfur Chem. 1973. V. 8. P. 27 – 29; Gmelin Handbook: F: PerFHalOrg.S., Springer-Verlag, Berlin-Heidelberg-New York-Tokyo, 1986, V. 2. P. 194.
Recommended for publication by Prof. V. E. Platonov
Fluorine Notes, 2018, 116, 3-4
