Received: November 2017
DOI 10.17677/fn20714807.2017.06.05
Fluorine Notes, 2017, 115, 9-10
Binuclear titanium(IV) chloride complexes with chiral tetraaryl-1,3-dioxolane-4,5-dimethanol ligands as a new type of catalysts of ethylene and propylene polymerization
S. Ch. Gagievaa), V.A. Tuskaev a),b), L.A. Rishina c), A.G. Buyanovskaya b), B. M. Bulychev a)
a)Department of Chemistry, M. V. Lomonosov Moscow State University, 1 Leninskie Gory, 199992 Moscow, Russian Federation. Fax: +7 (495) 932 8846. Email: sgagieva@yandex.ru
b)A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 28 ul. Vavilova, 119991 Moscow, Russian Federation. Fax: +7 (495) 135 5085
c) Institute of Chemical Physics, Russian Academy of Sciences, Moscow, Russian Federation.
Abstract: The mono- and binuclear coordination compounds of titanium (IV) stabilized by the new bi- and tetradentant chiral ligands (L) - (4R,5R)-2,2-dimethyl-,,','-tetraphenyl-1,3-dioxolane-4,5-dimethanol derivatives have been synthesized. These complexes, after activation with methylalumoxane, catalyze the polymerization of ethylene and propylene with corresponding activities: from 50 to 1000 kg PE (mol Ti h atm)-1 and from 50 to 200 kg PP (mole Ti h atm)-1. Replacement of phenyl moieties in ligands with perfluorophenyl leads to a significant increase in the catalytic activity of systems in the reactions of ethylene and propylene polymerization. It is shown that systems involving binuclear complexes and systems with complexes containing perfluorophenyl fragments in ligands produce the formation of polyethylenes with significantly higher molecular weights, in comparison with mononuclear and non-fluorinated counterparts.
Key words: titanium (IV), TADDOL, fluorine containing ligands, homogeneous catalysts, polymerization, polyethylene, polypropylene.
There has been an intensive growth of research in the synthesis of coordination compounds of 4-6 and 8-10 groups transition metals in the last two decades. Many of them proved to be effective components of catalytic olefin polymerization systems, called non-metalocene systems [1-6]. These catalysts are characterized by high activity and ability to produce various types of polyolefins: ultra-high molecular weight linear polyethylene (UHMWPE), low molecular weight PE with terminal vinyl groups, highly regular isotactic and syndiotactic PP, high molecular weight atactic polypropylene, ultrahigh-molecular weight statistical and block- copolymers of ethylene with propylene, copolymers of ethylene with norbornene and a number of other polyolefins [7-9]. One of the most interesting properties of some catalysts of this class is the ability to conduct "live" polymerization of ethylene and propylene even at high reaction temperatures, which makes it possible to obtain PE and PP with a very narrow molecular weight distribution (Mw/Mn ~ 1).
At the same time, systematic studies of complexes with chiral ligands containing perfluorinated substituents in the olefin polymerization are much rarer. These systems are interesting from two points of view: 1 - elucidation of the effects of a stronger electron withdrawing perfluorinated moieties in ligand's structure on the catalytic properties of the resulting complexes and hence on all polymers properties, include molecular weight, molecular-weight distribution, crystallinity, and so on; and 2- the ability to synthesize mono-, bi- and polynuclear complexes and to study the catalyst nuclearity effects on the activity and the properties of the polymer.
Bi- and polynuclear complexes of transition metals are effective catalysts for a variety of chemical reactions, including the polymerization of olefins [10]. In such structures the cooperative interaction between the proximate metal centers is possible, that lead to activities and selectivity that cannot be attained using the corresponding mononuclear counterparts. The cooperative effects in olefins polymerization catalysis can play a key role in modifying enchainment and chain-transfer kinetics which ultimately determines polymer morphology and the molecular weight up to the formation of ultra-high molecular weight polymers [11].
We have previously shown that titanium complexes with diol ligands are effective non-metallocene catalysts for ethylene polymerization [12-15]. In the present study the synthesis of a number of titanium (IV) complexes with a new 1,3-dioxalane-4,5-dimethanol derivatives are reported. Also the catalytic properties of these complexes in homogeneous reactions of ethylene and propylene polymerization have been investigated.
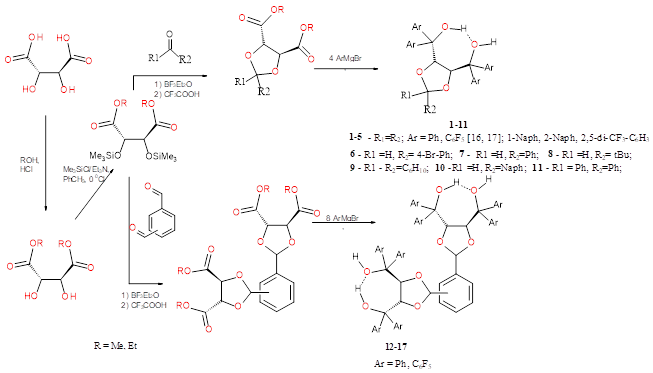
Chiral ligands 1-17 were obtained by the interaction of arylmagnesium bromides with the corresponding acetals of tartaric acid esters [16, 17] (Scheme 1). The introduction of sterically hindered aromatic substituents with different functional groups into the ligand structure makes it possible to change both the geometry of the complex, i.e. directly regulate the steric loading of the metal center, and its electronic characteristics. For these purposes fluorine atoms and trifluoromethyl groups are rather convenient substituents.
For the synthesis of ligands 12-17, which are able to coordinate two metal atoms, ortho-, phthalaldehyde, isophthalaldehyde and terephthalaldehyde were used as linkers between two chiral TADDOL fragments (Scheme 1).
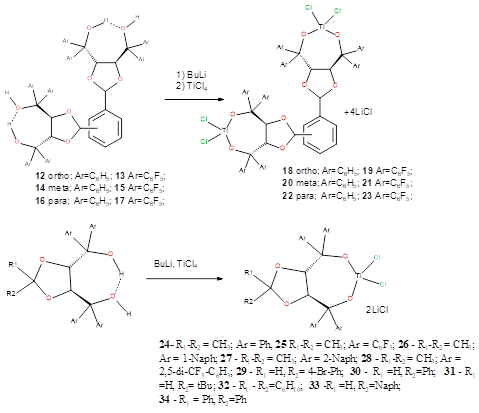
To obtain dichloride complexes of titanium with these ligands, we used three approaches: 1) interaction of the ligand with titanium (IV) dichloride diisopropylate followed by treatment with Me3SiCl; 2) through the stage of obtaining a spiro complex - (TADDOL)2Ti - and its subsequent treatment with TiCl4; 3) interaction of ligands, deprotonated by n-BuLi, with titanium tetrachloride. As a result, coordination compounds 18-34 were obtained.
Scheme 1
Scheme 2
The structures of these compounds are confirmed by elemental analysis, NMR spectra and mass spectroscopy. In the IR-spectra of complexes the appearance of stretching vibration bands of Ti-O and Ti-Cl in the range of 520-600 cm-1 were observed.
To study their catalytic properties, the complexes of the composition LTiCl2 or LTi2Cl4 were not separated from the by-product of the reaction - LiCl, but were immediately activated with polymethylaluminoxane (MAO) and used in ethylene polymerization in situ. The most significant results of this study are given in Table 1.
Table 1. Ethylene polymerization by 18-34/MAO catalytic systems a
|
No |
Comp- lex |
[Al]/ [Ti] |
Weight of PE, g |
A b |
Tm,c (°C) |
Degree of crystallinity d % |
Mw, 106 |
Mw/ Mn |
| 1 |
18 |
1000 |
0.29 |
192 |
138 |
47 |
1.50 |
2.7 d |
| 2 |
18 |
500 |
0.20 |
132 |
139 |
48 |
1.98 |
3.5 d |
| 3 |
18 |
3000 |
0.50 |
501 |
137 |
52 |
1.50 |
2.7 d |
| 4 |
19 |
500 |
0.35 |
240 |
142 |
58 |
3.7 g |
|
| 5 |
19 |
1000 |
0.70 |
463 |
143 |
69 |
5.64 g |
|
| 6 |
20 |
3000 |
0.12 |
212 |
138 |
52 |
1.45 |
|
| 7 |
20 |
1000 |
0.37 |
232 |
137 |
50 |
||
| 8 |
21 |
3000 |
0.36 |
230 |
140 |
73 |
4.68 g |
|
| 9 |
21 |
1000 |
0.79 |
510 |
142 |
70 |
||
| 10 |
22 |
1000 |
0.85 |
550 |
139 |
52 |
||
| 11 |
22 |
500 |
0.57 |
368 |
137 |
60 |
||
| 12 |
23 |
1000 |
1.50 |
968 |
143 |
77 |
4.25 g |
|
| 13 |
23 |
500 |
1.05 |
678 |
140 |
72 |
5.04 g |
|
| 14 |
24е |
3000 |
0.62 |
805 |
140 |
73 |
0.50 |
2.12 d |
| 15 |
24 |
1050 |
0.41 |
480 |
141 |
68 |
0.48 |
2.32 d |
| 16 |
24 |
200 |
0.12 |
21 |
142 |
54 |
||
| 17 |
25 |
1000 |
0.30 |
600 |
144 |
76 |
1.45 |
2.76 d |
| 18 |
25 |
500 |
0.24 |
480 |
143 |
79 |
1.68 |
2.67 d |
| 19 |
25 f |
1000 |
0.49 |
850 |
141 |
71 |
||
| 20 |
26 |
500 |
0.05 |
48 |
142 |
58 |
||
| 21 |
27 |
500 |
0.05 |
69 |
143 |
60 |
1.21 |
2.78 d |
| 22 |
28 |
500 |
0.10 |
120 |
139 |
62 |
||
| 23 |
29 |
500 |
0.12 |
150 |
138 |
68 |
1.05 |
3.93 d |
| 24 |
30 |
500 |
0.29 |
520 |
140 |
50 |
||
| 25 |
31 |
500 |
0.10 |
120 |
141 |
56 |
||
| 26 |
32 |
500 |
0.18 |
120 |
139 |
47 |
||
| 27 |
33 |
500 |
0.32 |
150 |
137 |
49 |
||
| 28 |
34 |
500 |
0.40 |
520 |
139 |
66 |
a Polymerizations were carried out in 10 mL of toluene at a constant ethylene pressure 0.7 atm., for 30 min, temperature 25 °C, С(Ti) = 1.7 10-6 mol;
b Activity, kg of PE (mol Ti atm)-1;
c Melting temperatures were determined by DSC (second heating);
d The degree of crystallinity were calculated from the DSC data using formula Hm100% = 288 J/g ) [18]; e The values of Mw were determined by the DSC
f the reaction was carried out in heptane
g Mw was measured by viscosimetry;
As can be seen from the table 1, all pre-catalysts 18-34 are more or less effective in ethylene polymerization reaction: the catalytic activity of systems varies in the range from 21 to 968 kg PE/(mole Ti h atm).
Catalytic systems based on the fluorine-containing binuclear complex 23 are the most effective (968 kg PE/(mole Ti h atm). An increase in the molar ratio AlMAO/Ti from 500 to 3000 mol/mol (for the precatalyst 18) is accompanied by a gradual increase in the activity of the catalytic system from 132 to 500 kg PE/mol Ti·h·atm.
Polymerization of ethylene on fluorine-containing binuclear TADDOL complexes 19, 21 and 23, in contrast to the phenyl analogs, leads to a significant increase of the catalytic activity (by 2-3 times). As can be seen from Fig. 2, the polymerization of ethylene on mono- and binuclear fluorine-containing complexes is characterized by relatively stable kinetics.
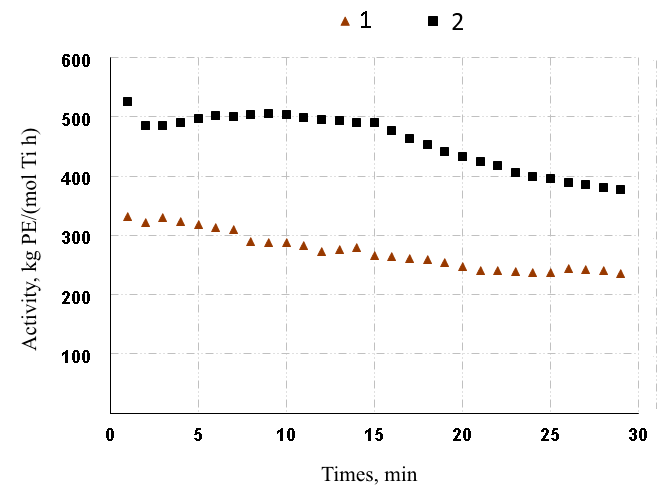
Figure 2. Kinetics of ethylene polymerization on precatalysts 23 (curve 2, run 12, table 1) and 25 (curve 1, run 17, table 1).
In the series of mononuclear complexes 24-34, precatalyst 25 is the most effective. As can be seen from Table 1, replacement of perfluorophenyl substituents by a bulky 1-naphthyl, 2-naphthyl and 2,5-di-CF3-phenyl moieties does not lead to an increase in the catalytic activity. The change in the nature of the substituents in the dioxolane fragment to tert-butyl, naphthyl, 4-bromophenyl also does not increase the activity (complexes 29, 31, 33). The introduction of one or two phenyl cycles into the dioxolane cycle (complexes 30 and 34) leads to an increase in the catalytic activity up to 520 kg PE/(mole Ti h atm) and only in the presence of two methyl groups in the dioxolane cycle (complexes 24, 25), the catalytic activity increases to 850 kg PE / (mole Ti h atm).
IR spectra of polyethylenes obtained on catalysts with TADDOL ligands are characterized by the presence of bands at 908 and 995 cm-1, which can be attributed to terminal vinyl groups. This indicates the chain transfer by -H elimination and the transition of hydrogen to Ti atom or to monomer [19]. The data of IR spectroscopy, TGA, DSC and viscosimetry (molecular weight up to 5 million) indicate that polymers have a predominantly linear structure and an ultrahigh molecular weight.
Relatively high stability of mononuclear fluorine-containing complex 25 and binuclear complexes 19, 21 and 23 under polymerization conditions, especially thermal stability of 25 at temperatures up to 50 °C, allowed to conduct the polymerization of propylene and the block copolymerization of propylene with ethylene.
On the mononuclear fluorine-containing titanium pre-catalyst 25, elastic polypropylene with isotactic inclusions (Tm = 150-156 °C, Tc = 4-5 °C and degree of crystallinity 9%) was formed [15]. On binuclear pre-catalysts 19, 23 the yield of polypropylene does not increase in comparison with the mononuclear analogue (Table 2), but in the NMR spectrum the proportion of isotactic sequences (mmmm) increases: 31.32, 27.75 (respectively).
Table 2. Propylene polymerization on 22,23,25/МАО a
|
No |
Complex |
C(Ti), 10-5 mol |
[Al]/[Ti] mol/ mol |
Polymer yield |
|
|---|---|---|---|---|---|
|
g |
kg/mol Ti |
||||
|
1 |
22 |
1.5 |
135 |
1 |
67 |
|
2 |
23 |
1.07 |
189 |
1 |
100 |
|
3 |
25 |
5.37 |
120 |
5 |
93.1 |
a Polymerizations were carried out in liquid propylene at a constant pressure, for 60 min, temperature 50 °C;
Table 3. Properties of polypropylene samples prepared with complexes 22, 23 and 25.
|
No |
Comp- lex |
D998/D973 |
D840/D973 |
D865/D1160 |
Тm, С |
Hm, J/g |
Тg, С |
Hg, J/g |
Degree of crystallinity, % |
|---|---|---|---|---|---|---|---|---|---|
|
1 |
22 |
0.445 |
0.30 |
0.0555 |
151.8 |
10.08 |
111.4 |
12.93 |
10.7 |
|
2 |
23 |
0.45 |
0.26 |
0.045 |
154.9/ 149.7 |
23.94/ 9.277 |
100.9 |
14.9 |
Amorphous |
|
3 |
25 |
0.47 |
0.27 |
0.0455 |
156.1/ 152.8 |
19.63/ 15.15 |
114.4 |
17.89 |
9 |
Melting temperatures were determined by DSC (first and second heating);
Table 4. 13C NMR stereoregularity data for PP produced with binuclear complexes 22, 23 and mononuclear complex 25.
|
No |
Complex |
Content of steric pentads, % |
||||||||
|
mmmm |
mmmr |
rmmr |
mmrr |
mrmm+ rmrr |
rmrm |
rrrr |
rrrm |
mrrm |
||
|
1 |
22 |
31.32 |
9.93 |
1.91 |
8.57 |
12.48 |
5.37 |
11.07 |
11.19 |
8.16 |
|
2 |
23 |
27.75 |
8.94 |
2.52 |
9.42 |
13.58 |
6.40 |
8.58 |
16.93 |
5.88 |
|
3 |
25 |
27.2 |
9.15 |
2.65 |
9.29 |
14.99 |
5.99 |
13.11 |
10.37 |
7.23 |
The introduction of perfluorophenyl moieties into the ligand structure leads to a marked increase in the catalytic activity of the systems, both in the polymerization of ethylene and propylene. This fact cannot be explained solely by steric factors. It is obvious that electronic effects play a big role, as well as the so-called "fluorine effect", which consists in the formation of weak non-covalent interactions between the fluorine atoms in the ligand and the hydrogen atoms of the growing polymer chain [9].
The activity of binuclear complexes either does not exceed or is substantially inferior to mononuclear analogs; however, polyethylene obtained on binuclear pre-catalysts is characterized by significantly higher MW values (up to 5.64 106 Da for complex 12), which suggests the existence of a cooperative interaction of two metal centers, preventing the chain termination process.
Experimental
All manipulations were performed under argon by using standard Schlenk techniques. Toluene, hexane and THF were distilled from Na/benzophenone prior to use [20]. Water contents of these solvents were periodically tested by Karl-Fischer coulometry with a Methrom 756 KF apparatus. Argon and ethylene of special-purity grade were dried by purging through a Super Clean™ Gas Filters. Methylaluminoxane MMAO-12 (Aldrich) was used as 7 wt % solution in toluene. Diisopropyl L- tartrate was prepared according to the previously described procedure [16]. 1,2-, 1,3- and 1,4-di-[(4R, 5R)-4,5-bis-(carboxyethyl)-1,3-dioxolan-2-yl]benzenes were prepared according to the procedure [21].
NMR spectra were recorded on Bruker АМХ-400 instruments. IR spectra were recorded on a Magna-IR 750 spectrophotometer. Elemental analysis was performed by the microanalytical laboratory at A. N. Nesmeyanov Institute of Organoelement Compounds.
Compounds 1-11 were obtained by the Grignard reaction according to the general procedure given in [22].
Synthesis of 1,2-bis[4,5-di(diphenyhydroxymethyl)-1,3-dioxolan-2-yl]benzene (12).
In an argon atmosphere, to a cooled to 0 ° C solution of Grignard reagent prepared from magnesium (0.94 g; 0.039 mole) and C6H5Br (6.0 g, 0.038 mmol) in 20 ml of tetrahydrofuran, solution of a 1,2-bis[4,5-di(carbethoxy)-1,3-dioxolan-2-yl]benzene (2 g, 3.9 mmol). After the addition was complete, the reaction mixture was heated to reflux for 2 hours, after that it was decomposed with 50 ml of saturated NH4Cl solution. The organic layer was separated, dried over Na2SO4, and the solvent was removed in vacuo. The resulting oil was recrystallized from toluene. Yield 0.50 g (90%). Тm 118°С; [α]D25 +1000 (s 1, CHC13). 1H NMR (CDCl3, δ, ppm.) 7.54-7.11 (m, 44Н, aromatic), 6.18 (s, 2Н, СН), 5.34 (d, 2Н, СН, J=4.0 Hz), 5.28 (d, 2Н, СН, J=4.0 Hz), 3.25 (s, 2Н, ОН), 1,90 (s, 2Н, ОН). UV-spectra (СН2Cl2), λmax, nm (lg ε): 245.2 (4.35), 253.6 (4.46), 259.4 (4.51). Found: С 80.67%; Н 5.65%. Calculated for С64Н54О8: С 80.82%; Н 5.72%.
Synthesis of 13-17 was carried out analogously to 12.
Synthesis of complexes 18-34 (general procedure).
A 2.5 M solution of butyllithium in heptane (0.42 mmol) was added dropwise with stirring under argon to a cooled (–78 °C) solution of ligand 1-17 (0.20 mmol) in toluene (10 mL). The temperature of the reaction mixture was slowly brought to ambient temperature, the mixture was stirred for 4 h and cooled to –78 °C. A solution of TiCl4 (0.20 mol) was added and the mixture was again warmed to ambient temperature. After 3 h, the reaction mixture was filtered, the solvent was evaporated, and the product was recrystallized from toluene:hexane = 1:1.
The polymerization of ethylene was performed in a thermostatically controlled reactor equipped with a magnetic stirrer and inlets for loading components of catalytic systems and ethylene at a total pressure of ethylene and solvent vapors of 0.7 atm. Toluene (10 ml) and the necessary amount of a co-catalyst methylalumoxane (MAO) were loaded in the reactor. The reactor was heated to a specified temperature, and the reaction mixture was saturated with ethylene. Polymerization was started by the addition of pre-catalyst to the reaction mixture. The pressure of ethylene was maintained constant during polymerization. Polymerization was stopped through the addition of 10% HCl solution in ethanol to the reactor. The polymer was filtered off, washed several times with water-ethanol mixture, and dried under vacuum at 70-80 °C until a constant weight was achieved.
Propylene polymerization reactions were carried out at 50 °C in liquid propylene; reaction conditions are given in Table 2. Details of the polymerization reactions were described earlier [15].
Polymer Evaluation Methods. TGA and DTA measurements were done by a “Derivatograph-C” (MOM, Hungary) at a heating rate 10oC/min in air. DSC was performed by a differential scanning calorimeter DSC-822e (Mettler-Toledo, Switzerland) at a heating rate 10oC/min in air. Viscosity-average molecular weight of synthesized samples was calculated with the Mark-Houwink equation: MW = 5.37·104 [η]1.37, where: MW = viscosity-average molecular weight (g/mol); [η] = intrinsic viscosity in decalin at 135°C (dl/g); [η]= (2ηsp -2lnηr )1/2/0.056 (ηsp - specific viscosity decalin at 135°C; ηr - relative viscosity in decalin at 135°C; ηr = ηsp +1.
13C NMR spectra of polymers (~ 5 wt.% solutions in o-dichlorobenzene) were recorded at 100 °C on a Bruker AVANCE-400 spectrometer (10.613 MHz).
Dedicated to the memory of T. Suchova.
Acknowledgments Financial support from the Russian Science Foundation (Project No 16-13-10502) is gratefully acknowledged.
References
- B.M. Trost, L.C. Czabaniuk. Angew. Chem. Int. Ed., 2014, 53, 2826 – 2851.
- M. C. Baier, M.A. Zuideveld, S. Mecking. Angew. Chem. Int. Ed., 2014, 53, 9722 – 9744.
- S. Wang, W.-H. Sun, C. Redshaw. J. Organomet. Chem., 2014, 751, 717-741.
- M.S. Khan, A. Haque, M. K. Al-Suti, P. R. Raithby. J. Organomet. Chem., 2015, 793, 114-133, DOI 10.1016/j.jorganchem.2015.03.023.
- L.C. So, S. Faucher, S. Zhu. Progress in Polymer Science, 2014, 39, 1196–1234.
- Y.-Y. Grace Luk, D.A. Foucher, R.A. Gossage. C. R. Chimie, 2013, 16, 573–579.
- Y.V. Kissin. J. Res.Updates Polym. Sci., 2013, 2, 8-24.
- Y.V. Kissin. Alkene Polymerization Reactions with Transition Metal Catalysts. Amsterdam: Elsevier 2008 [chapter 5].
- H. Makio, T. Fujita, Bull. Chem. Soc. Jpn. 2005, 78, 52–66.
- M. Delferro, T. J. Marks, Chem Rev., 2011, 111, 2450–2485.
- Ainooson M., Meyer F., in Comprehensive Inorganic Chemistry II (Second Edition), Volume 8: Coordination and Organometallic Chemistry, 2013, 433–458.
- M.V. Solov’ev, S.Ch. Gagieva, V.A. Tuskaev, N.M. Bravaya, O.E. Gadalova, V.N. Khrustalev, A.O. Borissova, B.M. Bulychev, Russ. Chem. Bull., 2011, 60, 2227-2235.
- L.А. Rishina, S.S. Lalayan, S.Ch. Gagieva, V.А. Тuskaev, A.N. Shchegolikhin, D.P. Shashkin, Y.V. Kissin, J. Res. Updates. Polym. Sci., 2015, 3, 216-226.
- Y.V. Kissin, L.A. Rishina, N.M. Galashina, S.Ch Gagieva, V.А. Tuskaev, Eur. Polym. J., 2009, 45, 2951-2961.
- L.A. Rishina, N.M. Galashina, S.Ch. Gagieva, V.A. Tuskaev, B.M. Bulychev, Yu N. Belokon, Polymer Science - Series A, 2008, 50, 110-118.
- Y. N. Belokon', S. C. Gagieva, T. A. Sukhova, Russ. Chem. Bull., 2005, 54, 2348–2353.
- V.A. Tuskaev, S.C. Gagieva, V.I. Maleev, M.V. Solov'ev, A.O. Borissova, Z.A. Starikova, B.M. Bulychev, Polymer, 2013, 54, 4455-4462.
- B. Wunderlich, C.M. Cormier, J. Polym. Sci Part A 2 Polym.Phys., 1967, 5, 987-988.
- L.K. Johnson, C.M. Killian, M.S. Brookhart, J. Am. Chem. Soc., 1995, 117, 6414.
- Organikum. Organischchemisches Grundpraktikum, VEB Deutscher Verlag der Wissenschaften, Berlin, 1990.
- D. Seebach, A. K. Beck, R. Imwinkelried, S. Roggo, A. Wonnacott, Helv. Chim. Acta, 1987, 70, 954.
- A. K. Beck, B. Bastani, D. A. Plattner, W. Petter, D. Seebach, H. Braunshweiger, P. Gysi, and L. LaVeccia, Chimia, 1991, 45, 238.
Recommended for publication by Prof. Sergey Igumnov
Fluorine Notes, 2017, 115, 9-10
