Received: October 2017
DOI 10.17677/fn20714807.2017.06.03
Fluorine Notes, 2017, 115, 5-6
IDENTIFICATION METHOD OF HEXAFLUOROPRYLENE DIMERS AND TRIMERS BY 19F NMR
Pospelova N. B. 1, Mokrushin I. G. 2
1Federal State Unitary Enterprise "Russian Scientific Center" Applied Chemistry",
2Perm State National Research University, Perm, Russia
Abstract The spectral characteristics of hexafluoropropylene oligomers are described and refined. The discrepancy between the chemical shifts of fluorine atoms was noted in the literature, and the refinement was carried out using the synthesis of model compounds. The mixtures of trimers in different ratios are analyzed. There was no significant concentration effect on chemical shifts. A method for determining the isomeric composition by NMR was proposed.
Keywords: nuclear magnetic resonance, NMR, fluorine-19, perfluorinated compounds, hexafluoropropylene.
Introduction
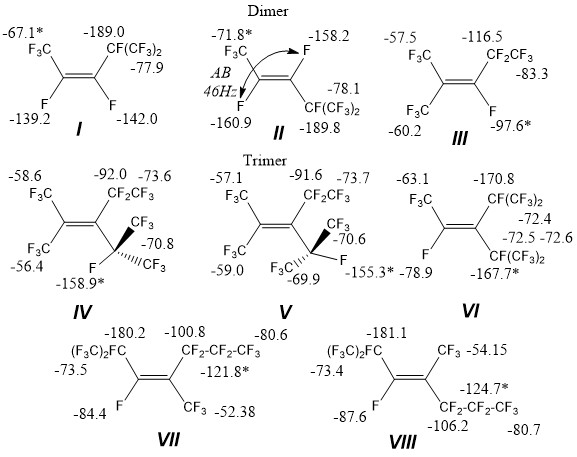
Perfluoropropene oligomers are used in production of surfactants as thermally stable solvents, refrigerants, inert liquids, dielectrics. Remote spin-spin interaction of fluorine atoms of spacious nature is known as characteristic for polyfluorinated molecules [1]. In this respect studying of atom 19F chemical shifts in mixtures close by molecule structure for example hexafluoropropylene I-VIII dimerization and trimerization products is of interest.
The special features of conducting the quantitative NMR analysis are described in manual [2]. The discrepancy of mole fraction may be less than 1 % when the conditions of qualitative record of spectrum are met. Weight fraction calculation methods by NMR can be described as estimates of integral intensity of all resonance lines of studied molecule and referring it to summary area of all spectrum lines according to formula 1. In order to make the calculation easier there are separated the “analytical” lines of each isomer (are marked with «*»), molar quantity is counted according to the formula 2.
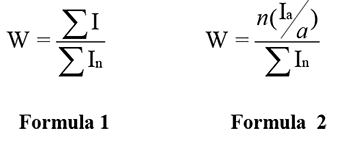
where ΣI is an integrated area of all the resonance lines of the studied molecule, Ia – is only analytical line’s area, a is the quantity of fluorine atoms that gave it, n is the total quantity of atoms in the determined molecule, ΣIn is the total integrated area of all resonance lines. [3]
Studying of Concentration Influence on Signal Position of Fluorine Resonance
We have studied NMR 19F spectra of hexafluoropropylene IV, V, VI trimers mixtures at their different ratios. Chemical shifts and mixtures compositions are listed in Table 1.
Table 1. Composition of HFP Trimers Mixtures And NMR 19F Signals Position (75.4 MHz)
|
Isomer |
Isomers |
Fluorine Chemical Shifts, ppm. |
|||||
|
Ratio, |
|||||||
|
% mole |
δ 1 |
δ 2 |
δ 3 |
δ 4 |
δ 5 |
δ 6 |
|
|
IV |
- |
159,8 |
87,7 |
71,2 |
70,3 |
59,1 |
56,1 |
|
62 |
158,3 |
86,7 |
70,5 |
70,2 |
58,6 |
55,6 |
|
|
44 |
158,4 |
87 |
69,9 |
* |
59,7 |
56,8 |
|
|
27 |
157,6 |
87,1 |
69 |
* |
59,3 |
56,9 |
|
|
V |
- |
156,1 |
91,5 |
74,1 |
70,3 |
52,1 |
56,4 |
|
23 |
154,6 |
91,6 |
70,5 |
70,2 |
58,6 |
57 |
|
|
28 |
154,2 |
91,9 |
69,9 |
* |
59,7 |
58,3 |
|
|
24 |
154,3 |
91,7 |
69 |
* |
59,3 |
57,2 |
|
|
VI |
100[3] |
180,8 |
180,2 |
78,9 |
73,2 |
72,9 |
13,3 |
|
15 |
* |
* |
* |
73,6 |
72,4 |
62,7 |
|
|
28 |
169,6 |
167,1 |
77,3 |
72,5 |
71,7 |
62,8 |
|
|
49 |
169,7 |
166,9 |
76,2 |
71,3 |
70,4 |
62,4 |
|
|
95 |
170,6 |
167,7 |
76,3 |
72,1 |
71,3 |
61,7 |
|
|
* - no precise referring of lines was done |
|||||||
As follows from the table, the measured chemical shifts are in good accordance with literature ones [4] practically for all fluorine atoms, they do not experience significant concentration influence and can be analyzed in mixture without dividing. Chemical shifts of fluorine atoms in positions 1 and 2 of isomer VI make the exception, they are moved into a weaker field for 10-14ppm relatively to the present ones [5]. Standard deviation from medium one is about 0.2-0.9 ppm for chemical shifts of NMR fluorine, e.g. up to 0,2% of a range and it is insignificant.
To make chemical shifts of isomer VI С4F and С3-СF fluorine atoms more precise we have used the fact, that interaction of trimer isomers’ mixtures and phenol or resorcin ultemately results in substitution of isomer VI C2F fluorine for phenyloxy-group [6] forming model compounds 2-aryloxyperfluoro(3-isopropyl-4-methylpenten-1) VIa and VIb:
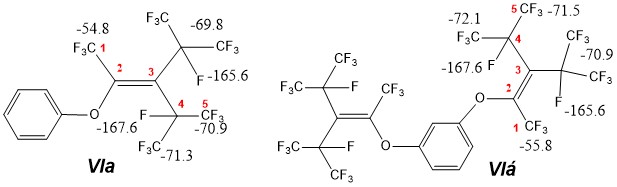
In NMR 19F VIa,b spectrum we observe a common tendency for moving of all absorption lines to a weak in relation to analogous lines VI field , that can be explained by inductive effect of aryloxygroup. Besides that, introduction of volume aryloxysubstituent resulted in increasing of CF3-group inequivalence in cis-position of isomer VI due to impromptness/freezing in its turning, that is confirmed by the presence of two lines - C5F and C4СF in spectrum (VIa, б).
Unresolved multiplet of fluorine 1 (in VI) can be explained by presence of complex effects of many interactions and exchange widenings caused by turning of transisopyl group in relation to methyl group. In VIa the structure of corresponding line is somewhat simplified due to exclusion of J1-3 interaction, but the form of line points out the presence of exchange processes between different rotameric positions, top temperature limit of which is higher than temperature of spectra taking.
Experimental Part
Isomers mixtures of total purity ~ 95% have been analyzed using spectrometers: Bruker WP80SY 80 MHz, with “capillary” with d-6 acetone, inner standard –hexafluorobenzene was added directly to the sample, temperature of recording/taking was 35°С; Bruker Avance III HD 400 MHz, with “capillary” with с DMSO -d6 + benzotrifluoride as external standard, temperature of recording/taking was 40°С. Standard in 19F spectra (ppm, in relation to CFCl3). Hexafluorobenzene - 162.90 ppm or benzotrifluoride -63.90 ppm. [3]
The composition of mixtures in Table 1 listed according to data of NMR was controlled using GLC. Isomer VI was isolated using preparative chromatograph ВАХВ-С8. Condition of isolation: column of 4m length, diameter-8 mm, sorbent - 20% 4F based on silanized silochrome С-80, fraction 0,315 – 0,5, columns temperature - 85°С, gas-carrier rate- (helium) 280 ml/min.
Oligomers (I-VIII) were obtained using known methods [2], “active” dimer HFP (III) was obtained by isomerization of mixture over catalyst. Chemical shifts of hexafluoropropylene I and II dimers were measured for mixture ~ 1:1.
Compounds VIa and VIб were obtained using known methods by interaction of hexafluoropropylene III trimer and phenol and resorcin over triethylamine respectively and purified by rectification. [4]
Dimers.
(Z)-1,1,1,2,3,4,5,5,5-nonafluoro-4-(trifluoromethyl)pent-2-ene (I).
19F NMR (376 MHz, DMSO-d6) δ -67.10 (dddhept, J = 36.0, 8.9, 8,3, 2.4 Hz), -77.85 (ddqd, J = 9.9, 7.6, 2.4, 0.8, Hz), -139.24 (qddhept, J = 8.3, 7.2, 4.4, 0.8 Hz), -141.98 (heptdqd, J = 9.9, 9.8, 8.9, 7.2 Hz), -189.04 (qdheptd, J =36.0, 9.8, 7.6, 4.6 Hz).
(E)-1,1,1,2,3,4,5,5,5-nonafluoro-4-(trifluoromethyl)pent-2-ene (II).
19F NMR (376 MHz, DMSO-d6) δ -71.84 (ddd, J = 23.0, 8.7, 0.7 Hz), -78.12 (ddd, J = 8.8, 7.8, 5.1 Hz), -158.18 (dqdhept, J = 139.5, 23.7, 9.8, 8.8 Hz), -160.89 (ddqhept, J = 139.5, 46.5, 8.5, 5.1 Hz), -189.76 (ddheptq, J = 46.5, 9.8, 7.8, 0.7 Hz).
1,1,1,3,4,4,5,5,5-nonanfluoro-2-(trifluoromethyl)pent-2-ene (III).
19F NMR (377 MHz, DMSO-d6) δ -57.49 (dtqq, J = 24.1, 20.1, 9.6, 4.7 Hz), -60.16 (dq, J = 30.4, 9.7 Hz), -83.33 (dq, J = 10.5, 4.7 Hz), -97.6 (qqqt, J = 30.4, 24.2, 10.5, 6.3 Hz), -116.45 (qd, J = 20.1, 6.3 Hz).
Trimers.
Rotamer A - 1,1,1,4,4,5,5,5-octafluoro-3-(perfluoropropan-2-yl)-2-(trifluoromethyl)pent-2-ene (IV). 19F NMR (376 MHz, DMSO-d6) δ -56.40 (dd, J = 52.5, 13.1 Hz), -58.63 (dh, J = 26.0, 13.1 Hz), -70.83 (qp, J = 13.5, 6.5 Hz), -72.48 (d, J = 1.0 Hz), -87.62 (dddt, J = 51.3, 41.2, 31.1, 10.6 Hz), -158.94 (dddt, J = 54.9, 51.8, 48.7, 3.0 Hz).
Rotamer B - 1,1,1,4,4,5,5,5-octafluoro-3-(perfluoropropan-2-yl)-2-(trifluoromethyl)pent-2-ene (V). 19F NMR (376 MHz, DMSO-d6) δ -57.11 (qddd, J = 19.7, 11.6, 8.0, 3.7 Hz), -58.98 (dpdd, J = 26.6, 15.0, 11.4, 3.5 Hz), -72.56 (dm, J = 27.5 Hz), -73.60 (ddq, J = 12.5, 6.3, 3.3 Hz), -73.69 (dddq, J = 9.8, 7.8, 5.7, 2.0 Hz), -91.65, -155.33 (tq, J = 39.2, 24.0 Hz).
1,1,1,2,4,5,5,5-octafluoro-3-(perfluoropropan-2-yl)-4-(trifluoromethyl)pent-2-ene (VI).
19F NMR (376 MHz, DMSO-d6) δ -63.05 (dhept, J = 52.8, 2.3 Hz), -72.40 (heptm, J = 4.3), -72.48 (d, J = 33.2 Hz), -72.56 (heptq, J = 4.3, 2.3 Hz), -78.09 (heptdd, J = 33.5, 9.2, 8.9 Hz), -167.7 (qdhept, J = 52.8, 9.2, 3.1 Hz), -170.78 (heptd, J = 30.9, 8.9 Hz).
((1,1,1,4,5,5,5-heptafluoro-3-(perfluoropropan-2-yl)-4-(trifluoromethyl)pent-2-en-2-yl)oxy)benzene (VIa). NMR 19F (pure), δ, ppm.: -54.8 (3F, C1F3, д, J= 58 Hz), -69.8 (6F, CF3, i-Pr, m), -70.9 (3F, C5F3, m), -71.3 (3F, C4-CF3, m), -165.6 (1F, CF, i-Pr, quart, J= 58 Hz), -167.6 (1F, C4F, hept, J= 35 Hz).
1,3-bis((1,1,1,4,5,5,5-heptafluoro-3-(perfluoropropan-2-yl)-4-(trifluoromethyl)-pent-2-en-2-yl)oxy)benzene (VIб). NMR 19F (pure), δ, ppm.: -55.8 (3F, C1F3, д, J= 58 Hz), -70.9 (6F, CF3, i-Pr, m), -71.5 (3F, C5F3, m), -72.1 (3F, C4-CF3, m), -165.6 (1F, CF, i-Pr, quart, J= 58 Hz), -167.6 (1F, C4F, hept, J= 35 Hz).
(Z)-1,1,1,2,3,5,5,6,6,7,7,7-dodecafluoro-2,4-bis(trifluoromethyl)hepta-3-ene(VII).
19F NMR (376 MHz, DMSO-d6) δ -52.38 (dd, J = 45.3, 10.7 Hz), -80.63 (td, J = 10.5, 3.7 Hz), --84.46 (tdtd, J = 34.7, 12.9, 7.3, 3.7 Hz), -106.17 (dqqtheptd, J = 49.1, 24.7, 9.9, 6.0, 4.1, 2.0, Hz), -124.74 (dqd, J = 17.4, 8.1, 2.0 Hz), -181.13 (dp, J = 18.8, 6.3 Hz).
(E)-1,1,1,2,3,5,5,6,6,7,7,7-dodecafluoro-2,4-bis(trifluoromethyl)hepta-3-en (VIII).
19F NMR (376 MHz, DMSO-d6) δ -54.15 (dt, J = 48.4, 11.5 Hz), 80.74 (td, J = 10.2, 1.6 Hz), -87.62 (tdt, J = 40.4, 16.8, 11.3 Hz), -100.82 (dqqtheptd, J = 69.0, 24.7, 10.2, 6.0, 4.1, 2.0 Hz), -121.79 (dqd, J = 33.3, 9.0, 3.8 Hz), -180.01 (dddd, J = 34.7, 22.5, 12.1, 6.1 Hz).
References
- Spektroskopiya yadernogo magnitnogo rezonansa vysokogo razresheniya: v 2h t. T. 1 / J. Emsly ; red.: V. F. Bystrov, Yu. N. Sheinker; per. s angl. B. A. Kvasov. M.: Mir, 1968. 630 s.
- Practical guide for quantitative 1D NMR integration. Eugenio Alvarado, University of Michigan, 2010. http://umich.edu/~chemnmr/docs/Quantitative_NMR.pdf
- Osobennosti NMR-analiza perftorirovannyh soedinenij. N.B. Pospelova, I.G. Mokrushin // Vestnik Permskogo universiteta, №3, 2016. DOI: 10.17072/2223-1838-2016-3-85-91
- Anionic oligomerisation of hexafluoropropene: fission of a carbon–carbon bond by fluoride ion // J. Chem. Soc. D, 1970, 1444-1446, DOI:10.1039/C29700001444
- Schumann C., Cavagna F., Non-bond F-F nuclear spin couplings II. Hexafluoropropene trimers. // J. Magn Reson., 1976; 22: 333-344.
- Patent SU 570591, 30.08.1977.
- R. R. Gupta, M. D. Lechner; Nuclear Magnetic Resonance (NMR) Data: Chemical Shifts and Coupling Constants for Fluorine-19 and Nitrogen-15 / Landolt-Börnstein - Group III Condensed Matter, Volume 35B, 1998. DOI: 10.1007/b55685
- Ishikawa, Nobuo; Nippon Kagaku Kaishi 1977, (10), P1411-15 CAPLUS
Recommended for publication by Prof. Sergey Igumnov
Fluorine Notes, 2017, 115, 5-6
