Received: September 2017
DOI 10.17677/fn20714807.2017.05.05
Fluorine Notes, 2017, 114, 9-10
Thermal Transformations of Polymer of Perfluoro-2,5-dimethyl-3,6-dioxanonanoic Acid Triethoxysilylpropylamide
Sakharov А.М., Popovich M. J., Krukovsky S.P.*
N.D. Zelinsky Institute of Organic Chemistry Russian Academy of Science,
117991, Moscow, Leninsky
prospect, 47,
e-mail: yar@ioc.ac.ru, as@zelinsky.ru
Abstract: Polymerization of perfluoro-2,5-dimethyl-3,6-dioxanonanoic acid triethoxysilylpropylamide (Fluorosam-39) was studied. It was found out that the polymer’s macromolecule contains two kinds of structure fragments, one of which takes part in the ramified parts of macromolecule formation, the other one – in the line sections of macromolecule formation. The polymer has an amorphous structure and does not dissolve neither in organic, nor in organofluorine solvents. It was stated that at thermal destruction the weakest point is an amide bond from which starts the polymer’s destruction, leading to the monohydro-derivative – 2H-heptadecafluoro-5-methyl-3,6-dioxanonane and a coke residue formation.
Keywords: triethoxysilylpropylamide of perfluoro-2,5-dimethyl-3,6-dioxanonanoic acid, polymer’s thermal destruction, monohydro-derivative – 2H-heptadecafluoro-5-methyl-3,6-dioxanonane.
Triethoxysilylpropylamides of perfluorooxaalkylenecarboxylic acids RFCOHN(CH2)3Si(OR)3, triethoxysilylpropylamide of perfluoro-2,5-dimethyl-3,6-dioxanonanoic acid (Fluorosam-39, F-39) particularly, were used [1-4] for hydro- and desensitization of chalk-stone, marble, plaster, brick, paper, wood and other materials.
The scheme of the synthesis of monomer F-39 from the ethyl ester of 2,5-dimethyl-3,6-dioxanonanoic acid* and (3-aminopropyl)triethoxysilane is given below:
RF CO(OC2H5)3 + H2N(CH2)3Si(OC2H5)3 → RF COHN(CH2)3Si(OC2H5)3
RF= CF3CF2CF2OCF(CF3) CF2OCF(CF3)-
*Ethyl ester used for obtaining of individual amide. For technical product production it is permitted to use methyl ester. In this case the product contain both methoxy- and ethoxy groups RfCONH (CH2)3Si(OCH3)x(OC2H5)3-x
The perfluoro-2,5-dimethyl-3,6-dioxanonanoic acid ethyl ester was treated with (3-aminopropyl)triethoxysilane (APTES) at 60 °С. The reaction path was controlled using the IR-spectroscopy for disappearance of the ester (1780 cm-1) and appearance of the amide group (1710 см-1). The obtained amide was distilled under vacuum, taking a fraction at the boiling point 110°С at 3-4 mm Hg.
The structure and contents of F-39 were based on elemental analysis data, IR, 1H and 19F NMR spectroscopic data.
Elemental analysis: found, %: С 30,78; H 3,23; N 1,97; F 45,98; Si 3,62; calculated: C18H22NF17SiO6., % :С 30,90; H 3,15; N 2,00; F 46,22; Si 4,00;
IR-spectra, υ, cm-1: 2979, 2932, 2890 (CH2); 3342, 3065 (NH);1749 (Amide 1); 1543 (Amide 2); 1243, 1159 (fluoroalkyl groups); 1105,1082 (Si-O-C).
1H NMR spectra, (CDCl3, δ, ppm): 0.74 (2H, SiCH2); 1,32 (m, 9H, OCH2CH3); 1,85 (m,2H, CH2); 3,45 (m, 6H, OCH2CH3); 8,28 (br.s, NH);
19F NMR spectra, (C6F6, δ, ppm): -81,4 - 85,73 (m, 13 F); -132 (m, 2F –CF2-); -134,28 (d, F, OCF(CF3)CF2- ); -147,30 (q, F, -OCFCO-).
Fluorosam-39 was easily dissolved in organic and organofluorine solvents. In general, it was used as 1-5% solution. When chalk-stone and other materials are treated with F-39 solution, then there is a interaction between its alkoxysilyl groups with reactive groups on the substrate’s surface (vaccination). Besides that, when there is a interaction with atmospheric moisture, there is also a polycondensation of F-39. As a result of these processes, on the surfaces of treated materials, the thinnest layer of F-39 forms, what gives the hydrophobic properties to the material.
The contact angles of water, engine oil 20W-30, decalin achieved 130° - 135°; 120° - 127° and 90-95° accordingly.
F-39 was also used as additive to oil-varnishes and enamels [5,6]. There were also developed F-39 water emulsions, in which perfluoro-2,5,8-trimethyl-3,6,9-trioxadodecanoic acid was used as emulsifier. This acid was obtained on the base of tetramer of hexafluoropropylene oxide [7]. The main attention was paid to the researches of the surface properties of treated materials. The properties of F-39 itself were not studied.
The Fluorosam-39 was dipped into a Petri dish made from polypropylene, than the dish was warmed in thermostat at 60-80°С to the constant weight. The loss of the weight by the sample was due to ethanol eliminating during polycondensation.
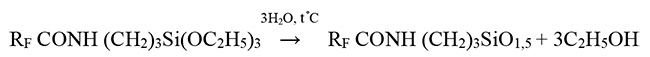
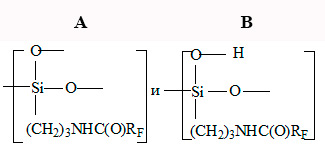
Hard, transparent, colorless, melting, practically insoluble polymer is formed while F-39 with atmospheric moisture (20-100°С) interaction. The macromolecule of such polymer, according to IR – spectroscopy and elemental analysis, seems to content basically two structure fragments: A and B
IR - spectra, υ, сm-1: 3466,3352, 3083 (NH); 2945, 2980 (CH2); 1707 (Amide 1);
1547 (Amide 2); 1239, 1154 (fluoroalkyl groups); 1071, 810 (-Si-O-Si-); 994 (-Si-OH).
Units A take part in the ramified parts of macromolecule formation, units B – in the linear part of macromolecule formation.
1,1,2-Trichloro-1,2,2-trifluoroethane (R-113) dissolve 2-10% of polymer. The remaining portion cannot be dissolved neither in organic, nor in organofluorine solvents. At the same time the polymer stays melting type, that means it is non-cross-linked.
The elemental analysis results of F-39 polymer and data, calculated for structures A and B are shown it the Table 1.
Table 1. The polymer F-39 elemental analysis and elemental composition, calculated for the structures А (C12H7F17SiO4,5) MW 588 and Б (C12H8F17SiO5) MW 597.
|
Element |
C,% |
H,% |
N,% |
F,% |
Si,% |
O,% by variety |
|
Found polymer F-39 |
23,98 |
1,76 |
2,21 |
54,10 |
4,69 |
13,26 |
|
Structure A |
24,49 |
1,19 |
2,38 |
54,93 |
4,76 |
12,25 |
|
Structure B |
24,12 |
1,34 |
2,34 |
54,10 |
4,69 |
13.41 |
According to the X-ray analysis data the polymer has an amorphous structure (two amorphous halo: 2θ ≈ 19º and 37º).
F-39 thermomechanical (TMA) curve is shown in the Picture 1.
Glass transition temperature of the polymer’s F-39 is 230°C, flow temperature is 270°C. At 45.6°C we can see one more phase change, which may take place due to the presence of low-molecular products in the sample. After law-molecular fraction is extracted with R113 (10%), then there is no such phase change.
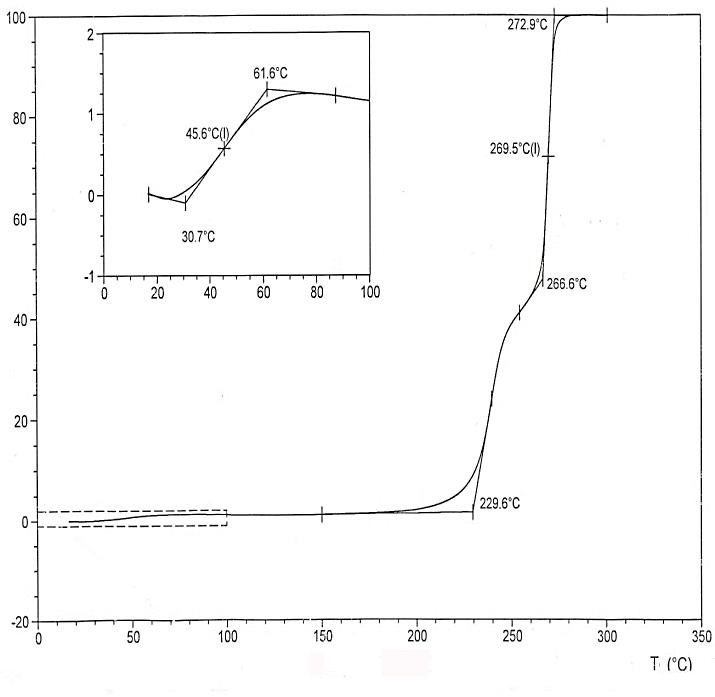
Pic. 1. F-39 thermomechanical curve.
According to the elemental analysis data and the results of thermomechanical researches, that allow to define the samples’ flow temperature, we draw a conclusion that in the macromolecules of obtained polymers there are more fragments from structure B.
The polymers thermal stability was studied by TGA method. In the Picture 2 there are shown the results of the polymer’s F-39 thermogravimetric analysis in the air and in inert atmosphere. The max decomposition rate is observed at 300°С. The reaction is exothermic in the air and endothermic in argon. At the temperatures higher than 300°C the decomposition rate slows down and between 500 - 600°C the sample’s weight stabilizes.
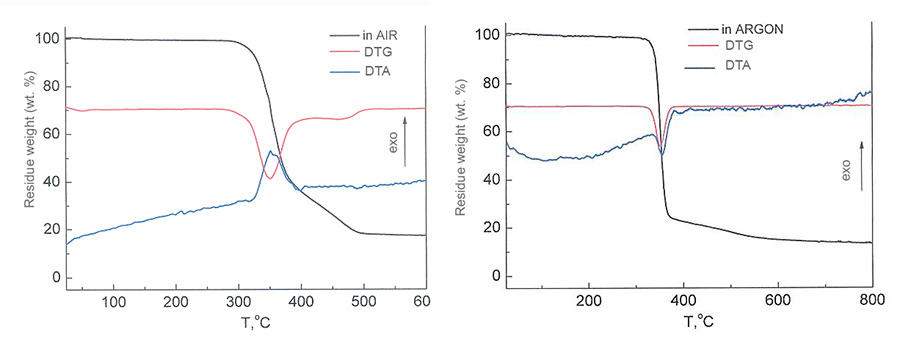
Pic. 2. F-39 TGA curves in air and argon.
The weight loss of sample in the air starts at the lower temperature (≈280°С), than in argon (≈350°С). When further heating at 350 - 400°C the weight loss speed in argon is higher than in the air. These probably happens because of the formation of more heat-resistant secondary structures when there is a cooperation between F-39 destruction products with atmospheric oxygen. At 470-700°C the weight losses become equal, achieving the rest maximum amount 12-14%.
Many works, devoted to the studies of thermal stability of siloxane polymers with structures, similar to A and B with different fluorinated substitutes, are mainly limited to the thermomechanical analysis. Given the data received, it was difficult to draw a conclusion about the mechanism of thermal decomposition and the character of decay products. Similar to that, it was not clear why at F-39 polymer’s thermal decomposition the samples’ weight loss in argon was much slowly than in the air. In order to study the mechanism of F-39 polymer’s thermal decomposition, it’s pyrolysis under vacuum at 340 °С was held.
In siloxanes with amide spacer the most sensible spot seems to be an amide bond, from which the polymer’s destruction starts according to the scheme:
RFCONH (CH2)3-[SiO1,5]x[Si(OH)O]n-x- → RFH + coke residue
Volatile destruction products, according to 1H and 19F NMR spectroscopic data and mass-spectroscopy analysis, mainly consisted of monohydro-derivative – 2H-heptadecafluoro-5-methyl-3,6-dioxanonane and ethanol. The quantity of the monohydro-derivative in the mixture was equal to 70-90%. Ethanol’s appearance can be explained by the presence of unreacted ethoxy groups in solidified polymer.
1H NMR, (CDCl3, δ, ppm): 5,96 , 6.14 (H,RFH)
19F NMR, (C6F6, δ, ppm): -82 - - 89 (m.13 F); -132.3(2F, m. CF3CF2CF2-); -146.98 (F, -OCF(CF3) CF2-); -148,45 (F, d. -CF(CF3)H)
Similar results were obtained for the polymers RFCONH (CH2)3-[SiO1,5]x [Si(OH)O]n-x-, where RF=CF3.
In the volatile destruction products, using mass-spectroscopy analysis method, it was found trifluoromethane CF3H.
Consequently, according to IR-, 1H and 19F NMR spectroscopic data, elemental analysis, TMA, TGA and mass-spectroscopy, it was found out that during of perfluoro-2,5-dimethyl-3,6-dioxanonanoic acid triethoxysilylpropylamide polymerization under the influence of atmospheric moisture, transparent, colorless, amorphous, non-cross-linked polymer is formed. This polymer has glass transition temperature 230°C and flow temperature - 270°C. Polymer’s macromolecule contains ramified and line fragments of the chain. The polymer does not dissolve neither in organic, nor in organofluorine solvents. The polymer’s thermal destruction starts with amide bond destruction which is followed by the formation of monohydro-derivative – 2H-heptadecafluoro-5-methyl-3,6-dioxanonane.
References
- Krukovsky S.P., Yarosh A.A., Glazkov A.A., Batizat D.V., Redina T.N.. «New fluorine-containing oligomers and polymers». J. Fluorine Chemistry, 96 (1999), p. 31-33
- Russian Patent 2149151, HYDRO-AND OLEOPHOBIC AGENT FOR PROTECTION OF BUILDING MATERIALS FROM ADVERSE EFFECT OF ENVIRONMENT, 2000
- Yarosh A.A., Krukovsky S.P., Pryakhina T.A., Kotov V.M., Zavin B.G. and Sakharov A.M. «Synthesis of water- and oil-repellent organofluorosilicon compounds» Mendeleev Commun., 2006 .p.190-192
- A. A. Yarosh, S.P. Krukovsky, A. M. Sakharov, M. Yu. Popovich, V. M. Kotov, T.A. Pryakhina, Synthesis and properties of fluorosilicon compounds for protection of cultural monuments from harmful environmental exposure, Russian Chemical Bulletin, Vol. 63, Iss. 2, 2014, pp 546–548.
- Russian Patent 2280653, METHOD FOR ALKYD RESIN PRODUCTION, 2006.
- Russian Patent 2280662, METHOD FOR PRODUCING LACQUER COATINGS, 2006.
- Russian Patent 2370476, HYDRO- AND OLEOPHOBIC AGENT FOR PROTECTING CONSTRUCTION MATERIALS AND STRUCTURES FROM DAMAGING ENVIRONMENTAL EFFECTS AND WATER-EMULSION COMPOSITION BASED ON SAID AGENT, 2009
Recommended for publication by Prof. S.P. Krukovsky
Fluorine Notes, 2017, 114, 9-10
