Received: August 2017
DOI 10.17677/fn20714807.2017.05.03
Fluorine Notes, 2017, 114, 5-6
Homo – and co-polymerisation of perfluoroisopropylvinyl ether under high pressure
E.V. Polunin1, S.I. Molchanova2, J. E. Pogodina 1, V.I. Sokolov2, I. V. Zavarzin 1
1N.D. Zelinsky Institute of Organic Chemistry Russian Academy of Science
2 Institute of Photon Technologies of Federal Scientific Research Centre «Crystallography and Photonics» of Russian Academy of Sciences
e-mail: polunin-507@yandex.ru
Abstract: Using the method of high pressure (1.1 – 1.2 GPa) without usage of initiator a homopolymer of perfluoroisopropylvinyl ether and it’s co-polymers with perfluoro-2-2-dimethyldioxole was obtained for the first time. The average molecular weight of the products was equal to 2 104 - 1.2 105 atomic mass unit (a. m. u., Da)
Keywords: perfluorinated homo- and co-polymers, polymerization under high pressure, thermal initiation, viscosimetry, refractive index.
The heighten interest in the new polymeric materials with high optical transmittance and low refractive index in an optical and a neighboring fields of spectrum is due to their high-demand in the high-speed optical transmission systems of information. Such polymers may be used as optical fiber covers and also for creation of information transferring optical busses on the base of the polymeric wave guides massive. For example for the microprocessing calculating devices on printed-circuit boards. Today the amorphous perfluorinated polymers are considered to be the best possible materials for these goals [1-4]. Opposite to its carbonic alternatives, such polymers have much lower absorption in all three “telecommunication” fields of wave length around 0.85, 1.3 and 1.5 μm. Perfluoropolymers have lower refractive index and lower material dispersion that identifies optical data transferring speed-limits.
The synthesis of homo-polymer of perfluoroisopropylvinyl ether 1 under high pressure and the radical polymerization thermal self-initiation was recently described [5], but in literature there was not found any information about the homo- and co-polymerization of its isomer, perfluoroisopropylvinyl ether 2. Probably it can be explained by the steric difficulties in a process of this isomer polymerization, which arise because of the presence of volumetric perfluoroisopropyl group close to the reactive center, containing C=C double bond.
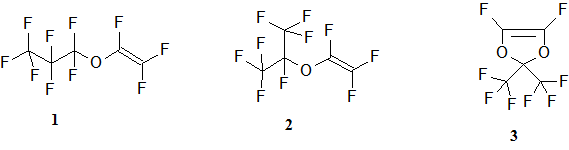
At the same time the homopolymer 2 and it’s co-polymers with the other perfluorinated monomers are of practical and theoretical interest. This is because the perfluoroisopropylvinyl and perfluoropropylvinyl ether’s homopolymers may have different refractive indexes n. However, a polymer with the higher n can be used for optical fibre core formation and a material with a lower n – for a cover creation. The difference between the refractive indexes of the homopolymers, produced from the monomers 1 and 2, which have the same number of C, F and O atoms in molecule and the same molecular mass, but the different structure, may be explained by the difference in the micromolecular chains packing density and as follows by the different free volume in the corresponding homopolymers. Similar ideas may be used for the co-polymers 1 and 2.
The high pressure method allowed us to get isomer’s 2 homopolymer and it’s co-polymers with perfluoro-2,2-dimethyldioxole 3 for the first time. The synthesis was realized on the Barostat equipment. It’s conceptual design was described in the article [6]. This equipment allows to carry synthesis under the pressure up to 1.5 GPa (≈ 15 000 atm) and the temperatures up to 350 0С. Previously the monomers were distilled in the argon atmosphere in order to remove the dissolved oxygen, which is known to be a radical polymerization inhibitor. Than the monomer 2 or the mixture of the monomers 2 and 3 in the given molecular ratio without the addition of the initiator was poured into a cylindrical teflon ampoule with a volume 1.5 cm3 . The ampoule was closed tightly with a Teflon lid and was put into the press-mold. In order to assure air-tightness of the reacting volume, sealing gaskets from a steel, copper and fluoroplastic were used. The pressure in the reacting ditch was equal to 1100-1200 MPa, the temperature – 150-170 0С, polymerization time was ranging from 9 to 20 hours. When the process was finished, the pressure in the reactor was smoothly lowered to the atmospheric pressure, the press-mold was cooled to 0-5 0С. After these the reaction mixture was put out and was held under vacuum 1 mm Hg at 70 - 90 0С until it got the constant weight.
The homopolymer 2: pressure 1200 MPa, 150оС, 20 hours, yield 27%. Co-polymer 4, produced from the monomers 2 + 3, in the molar ratio 1:1: 1100 MPa, 150 оС, 9 hours, yield 37%. Co-polymer 5 from 2 + 3 in ratio 1:4: 1200 MPa, 170 оС, 11 hours, yield 35%.
In 19F NMR spectrum of the polymerization products, that were measured on the Bruker AM-300, SF=282.40 MHz, there were signals, corresponding to its structure: -75 and -83 ppm (СF3-group); from -112 to -122 ppm (CF2-group) and from -141 to -144 ppm (OCF-group).
The dynamic viscosity of the obtained polymers was measured on the viscosimetry HVROC-S of the RheoSense company, USA. The characteristic viscosity [] was counted on the base of the Huggins' and Kraemer's equations. It was equal [] = 0.0077, 0.0486 and 0.0058 ml/mg for the homopolymer 2 and co-polymers 4 and 5 correspondingly, what allows to estimate its molecular weight as 2 104, 1.2 105 и 1.5 104 Da.
The perfluoroisopropylvinyl ether’s homopolymer refractive index 2 n = 1.3190, measured on the wave length = 632.8 nm, turned out to be higher than for the homopolymer of the perfluoropropylvinyl ether n = 1.3145 [7]. It is close to the minimal theoretical relevance n = 1.29 – 1.31, predicted in [2]. The differences in the refractive indexes of the homopolymers 1 and 2 can be explained by the characteristics of the polymer macromolecules package, produced from these isomers.
Acknowledgement
This research was supported by the Russian Foundation for Basic Research (RFBR) grant No 16-29-05407.
References
- N. Tanio, Y. Koike. What is the most transparent polymer? Polymer Journal, Vol. 32, No. 1, pp. 43 – 50, 2000.
- 2. W. Groh, A. Zimmermann. What is the lowest refractive index of an organic polymer? Macromolecules, Vol. 24, pp. 6660 – 6663, 1991.
- L. Eldada, L.W. Shacklette.>Advances in polymer integrated optics. IEEE Journal of selected topics in quantum electronics. Vol. 6, No. 1, pp. 54 - 68, 2000.
- M.K. Yang, R.H. French, E.W. Tokarsky. Optical properties of TeflonAF amorphous fluoropolymers. J. Micro/Nanolith. MEMS MOEMS, Vol. 7(3), pp. 033010-1 – 033010-8, 2008.
- A. A. Zharov, I. B. Konovalova, E. V. Polunin. Synthesis of amorphous homopolymer of perfluoropropyl vinyl ether at a high pressure. Russian Chemical Bulletin, International Edition, Vol. 65, No. 1, pp. 233—236, January, 2016.
- A. A. Zharov, I. A. Guzyaeva. Kinetics and mechanism of thermal polymerization of hexafluoropropylene under high pressures. Russian Chemical Bulletin, International Edition, Vol. 59, No. 6, pp. 1225—1231, June, 2010.
- V.I.Sokolov, V.E.Boyko, I.O.Goryachuk, S.M.Igumnov, S.I.Molchanova, Yu.E.Pogodina, E.V.Polunin. Synthesis and optical properties of copolymers of perfluoro-2,2-dimethyl-1,3-dioxole and perfluoropropyl vinyl ether. Russian Chemical Bulletin, International Edition, Vol. 66, No. 7, pp. 1284—1289, July , 2010.
Recommended for publication by Prof. S.M. Igumnov
Fluorine Notes, 2017, 114, 5-6
