Received: August 2017
DOI 10.17677/fn20714807.2017.05.02
Fluorine Notes, 2017, 114, 3-4
PRODUCTION OF HYDROXYAPATITE BASED FLUORINE-CONTAINING COMPOSITE MATERIALS
Bogdanova Е.А., Skachkova О.V., Skachkov V.М., Sabirzyanov N.А.
Federal State government-financed institution of science Institute of Solid State Chemistry, Ural Branch of the Russian Academy of Sciences,
91 Pervomaiskaya str., 620990, Ekaterinburg (Russia)
е-mail: chemi4@rambler.ru
Abstract. Mechanochemical synthesis of composite materials based on hydroxyapatite and calcium fluoride (10, 15, 20 wt.% CaF2) has been carried out in a vibratory mill with simultaneous mixing and grinding of the initial components, followed by annealing in the temperature range 200-1000°C. The features of the chemical interaction of HAP with a reinforcing additive during heat treatment, its effect on the grain size, the change in properties and structure during heating and annealing are revealed. The effect of the phase composition and the amount of the additive on the strength characteristics of the test samples is shown. The optimum amount of CaF2 reinforcing agent (15 wt.%), which ensures the formation of a dense and strong composite material has been chosen.
Keywords: hydroxyapatite, fluorapatite, composite materials, microhardness
Introduction
In medical practice, for the replacement and restoration of bone tissues, extensive use is made of biomaterials based on calcium phosphates, mainly Ca10(PO4)6(OH)2 (hydroxyapatite HAP), which has a structure and chemical composition almost identical to natural bone tissues and exhibits a pronounced osteotropic behavior in biological media [1-3]. Calcium phosphates based bioceramic materials enjoy special popularity. The drawback of HAP based bioceramics is a low mechanical strength, which prevents their application for the elimination of defects of bone tissues experiencing regularly considerable mechanical loads.
The strength and fracture toughness of HAP based biomaterials can be enhanced by chemical modification of the structure, since the characteristics of a material can be varied in a wide range by changing the number and character of surface functional groups, as well as by mechanical synthesis of crystalline HAP with a reinforcement additive (CaF2, SiO2, TiO2, ZrO2, Al2O3 etc.). When such composite materials are produced, HAP interacts with the reinforcing phase during heat treatment, and the phase composition of the components can be changed. This requires the examination of the physicochemical processes taking place in the corresponding systems, the effect of the type and amount of additive on the microstructure, phase composition and mechanical characteristics of materials, as well as the biomedical properties.
In this work, the structure and properties of HAP based ceramic materials with calcium fluoride additives forming the system Ca10(PO4)6(OH)2CaF2 have been studied, and the peculiarities of the chemical interaction of HAP with the reinforcement additive during thermal treatment, its effect on the grain size, properties and structure variation during heating and annealing have been revealed.
Experimental
The studies were conducted on a series of 4 samples. Sample 1 was prepared in the following way: 13.5 g (0.013 mol) of HAP of the composition Ca10(PO4)6(OH)2 [4] and 1.5 g (0.02 mol) of CaF2 (chemically pure, Spec 6-09-01-572-79, Vekton, Russia) were mixed in a vibratory mill (MLW 4000 KM 1) with an agate mortar and a ball for 30 min without addition of a homogenizer. As a result of mechanical synthesis, 15 g of the composite material Ca10(PO4)6(OH)2CaF2 containing 90 wt.% of HAP and 10 wt.% of CaF2 have been produced. In the same way we obtained 15 g of sample 2 containing 85 wt.% (12.75 g, 0.013 mol) of HAP and 15 wt.% (2.25 g, 0.03 mol) of CaF2 and also 15 g of sample 3 containing 80 wt.% (12 g, 0.012 mol) of HAP and 20 wt.% (3 g, 0.04 mol) of CaF2. Sample 4 – the initial HAP of the composition Ca10(PO4)6(OH)2 [4] – was studied as a reference specimen.
The samples were annealed in a Nabertherm L 9/11 muffle furnace in the temperature interval 2001000°C with a step of 200°C at a heating rate of 10°C/min. The X-ray diffraction analysis (XRD) was performed on Shimadzu and DRON-2.0 diffractometers in CuK radiation with the angle interval 10 2 70, a scanning step of 0.03° and 2 s time in point. The phases were identified with the use of the Powder Diffraction File JCPDSD-ICDD PDF2 (sets 1-47). The microhardness of the composite materials was measured by the Vickers method on a PMT-3М microhardness tester with a load of 0.98 Н (100 g) and loading time 10 s. The specific surface area was determined by the Brunauer-Emmett-Taylor (BET) method of low-temperature nitrogen adsorption on a Gemini VII 2390 V1.03 (V1.03 t) automatic surface and porosity analyzer (Micromeritics). Preliminarily, degassing of samples was carried out at T=200C for 1 h on a Sample Degas System VacPrep 061 (Micromeritics). The morphological features were studied by the scanning electron microscopy (SEM) method on a JEOL JSM 6390 LA microscope (Japan) having the magnification factor from х5 to х300000 and the resolving power 3.0 nm at 30 kV. The particle size was determined on a universal particle size distribution laser express analyzer Horiba LA-950 in the measurement range from 0.01 μm to 3000 μm with the maximum error 0.6 %.
Results and Discussion
Mechanochemical synthesis of composite materials Ca10(PO4)6(OH)2CaF2 (10, 15, 20 wt.% CaF2) has been performed in this work by concurrent mixing and grinding of the initial components in a mill with their subsequent annealing in a wide temperature range (200-1000С). Mechanochemical activation allows the fine dispersion of the components to be increased, which is one of the ways to achieve a high strength. Besides, bond rupture takes place in the process of activation giving rise to new chemical compounds as a result of mechanochemical reactions [5].
In case of the Ca10(PO4)6(OH)2CaF2 system, mechanical activation and subsequent heat treatment lead to the formation of a new fluorapatite (FA) phase of the composition Ca10(PO4)6F2 at temperatures above 200C, which is confirmed by the XPA data (Table 1).
The introduction of calcium fluoride affects the behavior of samples during annealing. It is known that HAP produced by deposition from solutions partially decomposes forming Са3(РO4)2 tricalcium phosphate (TCP) already at 800С [6, 7]. The presence of CaF2 in the composition of the sample prevents the decomposition of HAP to calcium phosphate and makes it possible to stabilize the material to the decomposition temperatures typical of HAP produced by solid-phase synthesis (Table 1). The variation in the phase composition during annealing is influenced not only by the presence of calcium fluoride in the sample, but also by its amount. So, an increase in the CaF2 content to 20 wt.% (sample 3) results in complete transformation of HAP to FA at 800°C, and further heat treatment promotes the decomposition of the apatite phase into calcium phosphate
Table 1. XPA results for composite materials Ca10(PO4)6(OH)2CaF2
|
Samples |
Phase composition at different temperatures |
|||||
|
25°C |
200°C |
400°C |
600°C |
800°C |
1000°C |
|
|
Sample 1 |
CaF2 HAP |
CaF2 HAP FA |
CaF2 HAP FA |
CaF2 HAP FA |
CaF2 HAP FA |
CaF2 FA |
|
Sample 2 |
CaF2 HAP FA |
CaF2 HAP FA |
CaF2 HAP FA |
CaF2 HAP FA |
CaF2 HAP FA |
|
|
Sample 3 |
CaF2 HAP FA |
CaF2 HAP FA |
CaF2 HAP FA |
CaF2 FA |
CaF2 FA TCP |
|
With a rise in the annealing temperature, the crystallinity degree of the samples increases, as evidenced by improved resolution and reduced width of the peaks on the X-ray diffraction patterns. According to the obtained SEM images (Fig. 1), the samples annealed at 600С are weakly crystallized. Further annealing above 800С leads to compaction of the material owing to removal of isolated pores and recrystallization, the grain size being from 0.4-0.9 μm (800С) to 1-4 μm (1000С). The SEM data confirm the formation of large columnar FA crystals with a sharp hexagonal faceting during annealing of Ca10(PO4)6(OH)2СаF2 composites.
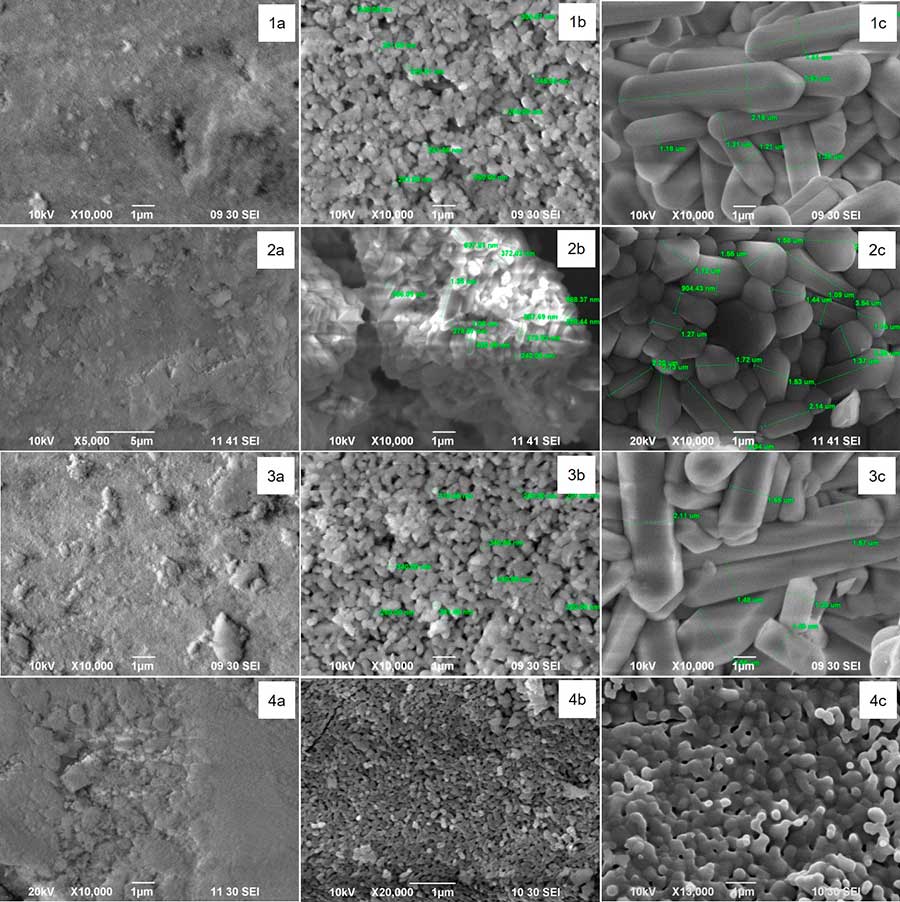
Fig. 1. The morphology of initial substances and Ca10(PO4)6(OH)2CaF2 composites: 1 - sample 1: а - 600C; b - 800C; c - 1000C; 2 - sample 2: а - 600C; b - 800C; c - 1000C; 3 - sample 3: а - 600C; b - 800C; c - 1000C; 4 - sample 4: а - 600C; b - 800C; c - 1000C.
The morphological data obtained by the SEM method agree with the results of particle size distribution measurements by the laser diffraction method (Fig. 2), according to which the initial powders have a rather wide particle size distribution range from 1 μm to 15 μm (the average particle diameter is 1.8 μm). During the interaction in the system Ca10(PO4)6(OH)215 wt.% CaF2 (sample 2) giving rise to FA, a more uniform particle distribution takes place in the process of annealing when the particle diameter decreasing to 1 μm. For HAP (sample 4), a monotonous grain growth occurs during annealing to 800C, and the particle size reduction at further heat treatment is due to structural transformations: removal of OH groups and formation of calcium phosphate.
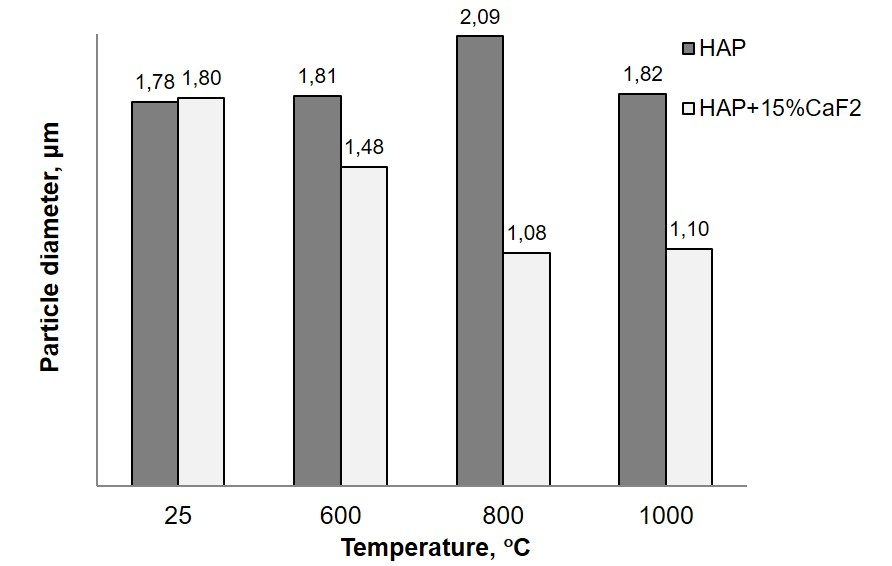
Fig. 2. Particle size distribution of Ca10(PO4)6(OH)215 wt. % CaF2 composite (sample 2) and Ca10(PO4)6(OH)2 (sample 4) vs the annealing temperature.
The estimation of the linear parameters of examined samples allowed us to establish the dependence of the linear shrinkage of the sintered materials on their composition and the annealing temperature (Fig. 3). The compaction of the composite materials Ca10(PO4)6(OH)2СаF2 begins at 600°C and reaches the maximum at 800°С. In the considered temperature interval (25-1000C), sample 2 containing 15 wt.% СаF2 has the largest density at the minimal mass loss (Table 2).
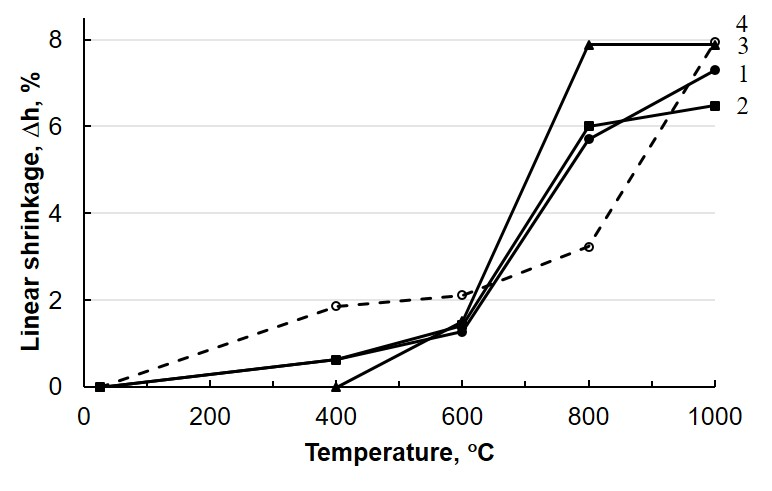
Fig. 3. The linear shrinkage of composite materials Ca10(PO4)6(OH)2CaF2 vs the annealing temperature: 1 - sample 1, 2 - sample 2, 3 - sample 3, 4 - sample 4.
Table 2. The mass loss of Ca10(PO4)6(OH)2CaF2 composites vs the temperature
|
Samples |
Mass loss, % |
|||
|
400°C |
600°C |
800°C |
1000°C |
|
|
Sample 1 |
4.43 |
5.51 |
7.30 |
8.80 |
|
Sample 2 |
3.72 |
6.00 |
6.70 |
6.70 |
|
Sample 3 |
3.80 |
4.97 |
6.50 |
8.55 |
|
Sample 4 |
5.50 |
6.89 |
8.99 |
9.48 |
The variations in the specific surface area and porosity of the materials during compaction were estimated by the BET method (Table 3). As the annealing temperature is raised, the surface becomes less developed; at 1000C the samples are sintered and the pores are absent. The presence of pores for the composite Ca10(PO4)6(OH)220 wt.% СаF2 (sample 3) and pure HAP (sample 4) can be explained by surface “burning” during decomposition of the apatitie phase.
Table 3. The results of measurements of the specific surface area and porosity of samples
|
Samples |
Specific surface area and porosity, m2/g |
||
|
25°C |
800°C |
1000°C |
|
|
Sample 1 |
82.2395±0.4981 |
2.7823±0.0551 |
– |
|
Sample 2 |
77.3967±0.4532 |
0.8233±0.0166 |
– |
|
Sample 3 |
71.6385±0.4066 |
4.2809±0.0367 |
0.5191±0.0210 |
|
Sample 4 |
98.8021±0.6497 |
12.1417±0.1236 |
0.4359±0.0126 |
|
Pore area, m2/g |
|||
|
Sample 1 |
7.7771 |
1.4198 |
– |
|
Sample 2 |
8.0683 |
0.3384 |
– |
|
Sample 3 |
7.0771 |
0.3620 |
|
|
Sample 4 |
10.3677 |
0.7829 |
0.3455 |
|
Pore volume, cm3/g |
|||
|
Sample 1 |
0.004040 |
0.000833 |
– |
|
Sample 2 |
0.004211 |
0.000204 |
– |
|
Sample 3 |
0.003677 |
0.000208 |
|
|
Sample 4 |
0.005483 |
0.000474 |
0.000197 |
Compaction of the material in the process of sintering and formation of fluorapatite phase during the interaction of HAP and calcium fluoride increases the strength of the composite material (Fig. 4). Comparison of the microhardness measurement (Fig. 4) and XPA (Table 1) results allows us to conclude that the strengthening of the composite is due not only to the formation of the FA phase during annealing, but also to the simultaneous presence of HAP and FA in the composition of the sample. Partial decomposition of the apatite phase to calcium phosphate typical of both pure HAP [6, 7] and sample 3 containing 20 wt.% СаF2 decreases the microhardness of the material.
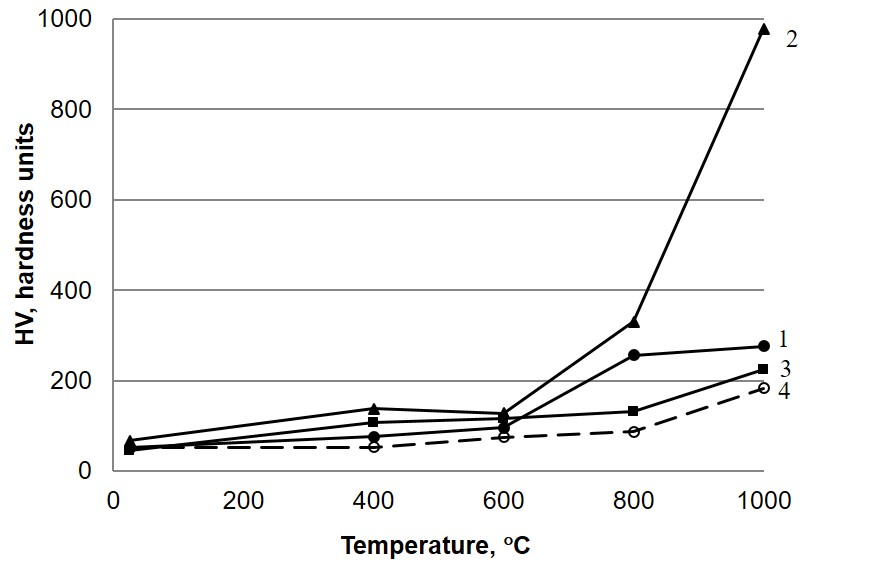
Fig. 4. The microhardness of the samples vs the annealing temperature: 1-sample 1; 2 - sample 2; 3 - sample 3; 4 - sample 4.
As a result of the work, it has been established that mechanochemical activation and annealing leads to the interaction in the Ca10(PO4)6(OH)2CaF2 system with the formation of the fluorapatite phase at a temperature above 200C. A strong composite material having a uniform compact structure with a high degree of crystallinity, stable at 1000°C, was obtained by introducing of 15 wt% CaF2. Increasing the strength of this sample is facilitated by the formation of FA and its simultaneous presence with HAP in the material.
The formation of the FA and its simultaneous presence with HAP is also characteristic for a sample containing 10 wt% CaF2. However, the amount of reinforcing agent in this case is insufficient to achieve the necessary strength characteristics at temperatures above 800°C. An increase in the content of CaF2 in the material up to 20 wt% promotes the complete transition of HAP to FA at 800°C and the beginning of decomposition of the apatite phase into calcium phosphate at 1000°C. The structural disorder accompanying these phase transformations leads to a decrease in the microhardness of the material.
The results obtained agree with the regular features of ceramic materials described in the literature.
References
- Bezrukov V. М. Hydroxyapatite as a substrate for bone grafting: theoretical and applied aspects of the problem / V. М. Bezrukov, А. S. Grigoryan // Stomatologiya. – 1996. – V. 75. – No. 5. – P. 7-12. [in Russian]
- Akopyan G. V. The use of osteoplastic materials in dental implantology / G. V. Akopyan, А. G. Khachatryan // Assotsiatsiya stomatologov v Armenii. Theoretical and practical journal. – 2012. – V. 7. – No. 1. – P. 10-14. [in Russian]
- Stroganova Е. Е. Novel technologies for production and application of bioceramics in restorative medicine / Е. Е. Stroganova // Steklo i keramika. – 2008. – No. 1. – P. 36-38. [in Russian]
- RF Patent 2406693, МПK C01B 25/32 Method for production of hydroxyapatite suspension / N. А. Sabirzyanov; applicant and rightholder – Institute of Solid State Chemistry of the Ural Branch of RAS; publ. 20.12.2010, Bull. No. 35. [in Russian]
- Boldyrev V. V. Mechanochemistry and mechanical activation of solid substances / V. V. Boldyrev // Uspekhi khimii. – 2006. – V. 75. – No. 3. – P. 203-216. [in Russian]
- Barinov S. М. Bioceramics in medicine / S. М. Barinov, V. S. Komlev. – Moscow: Nauka, 2005. – 284 p. [in Russian]
- Bogdanova Е. А. Investigation into thermal stability of fluorine-substituted HAP / Е. А. Bogdanova, N. А. Sabirzyanov // Materialovedeniye. 2015. No. 1. P. 52-56. [in Russian]
Recommended for publication by Prof. S.M. Igumnov
Fluorine Notes, 2017, 114, 3-4
