Received: June 2017
DOI 10.17677/fn20714807.2017.04.04
Fluorine Notes, 2017, 113, 7-8
2,5-Furandicarboxylic Acid Fluoro-Containing Esters Synthesis
А.М. Sakharov, O.U. Smirnova, A.А. Yarosh
N.D. Zelinsky Institute of Organic Chemistry Russian Academy of Science,117991, Moscow, Leninsky prospect, 47,
e-mail: as@zelinsky.ru, yar@ioc.ac.ru
Abstract: The Fluoro-containing esters based on 2,5-furandicarboxylic acid as well as on its derivatives and 2,2,3,3,4,4,5,5-Octafluoropentan-1-ol (fluorotelomer alcohol n-2) have been synthesized. Sulphuric acid, tetrabutoxytitanium and potassium bisulphate were used as etherification catalysts. The reaction was controlled by NMR- 1Н, 19F and 13С. It was proved, that tetrabutoxytitanium partial re-esterification occurred when using it at 125°С due to the fact, that 2,2,3,3,4,4,5,5-octafluoroamyloxy-group is replacing butoxy-group. The best results have been achieved when carrying out the reaction using of 2,5-furandicarboxylic acid dichloroanhydride.
Key words: 2,5-Furandicarboxylic acid, 2,2,3,3,4,4,5,5-Octafluoropentan-1-ol, Sulphuric acid, Tetrabutoxytitanium, Potassium bisulphate, 2,5-Furandicarboxylic acid dichloroanhydride, NMR-spectroscopy.
Fluoro-containing esters synthesis is usually carried out proceeding from anhydrides, choloroanhydrides, esters of fluorine containing acids and fluorine containing alcohols. The reaction is carried out under the conditions of acidic catalysis, the yield of esters reaches 73% [1,2]
2,5-Furandicarboxylic acid (FDCA), which is a product of oxidation of 5-hydroxymethylfurfural (5-HMF) – one of the main synthons of modern organic synthesis was used as aliphatic carboxylic acid. While boiling the corresponding acid with methanol over sulphuric acid as catalyst [2] dimethyl ester ( >DMEFDCA) was preliminarily obtained for the synthesis of fluoro-containing esters. The yield of target product amounted to 55%. The structure of DMEFDCA has been confirmed by NMR- 1Н and 13С spectra which correspond to literature data (Scheme 1):
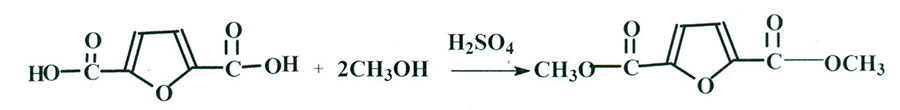
Scheme 1
Several target compound – fluorine containing ethers of 2,5-furandicarboxylic acid -synthesis paths have been studied (Schemes 2-5):
Using the reaction of DMEFDCA re-esterification when it is interacting with 2,2,3,3,4,4,5,5-octafluoropentan-1-ol (Scheme 2):
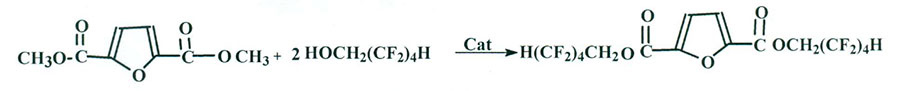
Scheme 2
H2SO4 , СF3СООН were used as catalysts of re-esterification (according to Scheme 2), as well as Ti(OC4H9)4 (TBT) [2-4]. In the last case DMF was used as solvent. The reaction was controlled according to data of NMR- 1 Н and 19F. No re-esterification reaction products were found over sulphuric and trifluoroacetic acids.
The formation of target product was observed while carrying out the reaction of DMEFDCA with 2,2,3,3,4,4,5,5-octafluoropentan-1-ol taken at a mole ratio 1:2 and TBT as catalyst (mole ratio DMEFDCA:TBT=3:1) in the solution of DMF. Using NMR 19F spectra was the most convenient way of controlling re-esterification comparing integral signal intensities at -127.5 ppm (OCH2CF2 group in the initial alcohol) and -121 .8 ppm for group(COOCH2CF2) in the reaction product. Besides that, the signals appeared at -127.2 and -132.3 ppm while signals preserving at -127.5 and -132.3 ppm, typical for 2,2,3,3,4,4,5,5-octafluoropentan-1-ol which had not reacted. NMR- 1Н spectra are characterized by signals presence in the area of 7.23 ppm (protons of furan ring) and 3.9 ppm (СН3OOС from DMEFDCA) . Comparing of their integral intensities were in accordance with NMR- 19F spectral data.
A suggestion was made, that in the current reaction TBT acts not only as catalyst, but also enters into re-esterification reaction with 2,2,3,3,4,4,5,5-octafluoropentan-1-ol at 125 °С.
The reaction between TBT and 2,2,3,3,4,4,5,5-octafluoropentan-1-ol was carried out at 125 °С and at a mole rate of reagents 1 : 2 for 3 hours to find out the that particular issue. In NMR- 19F spectrum two duplets appear in the area of -127.1- 128.2 and -131.3- 1 132.7 ppm. The signals at -122.7 and -139.1 typical for signals of CF2CF2 groups positioned in the middle of 2,2,3,3,4,4,5,5-octafluoropentan-1-ol molecule chain remain their places.
From the data of NMR- 13С spectrum [ppm:103-120 [5 triplets from groups (CF2)4; 74.9 (СН2О); 34.0, 17.8 and 12.2 for СН2СН2СН3 groups as in TBT] we can make a conclusion, that at 125 °С at least one butoxy-group in TBT (butanol b.p. is 117 °С) is replaced by alkoxy-group of 2,2,3,3,4,4,5,5-octafluoropentan-1-ol. Thus, the conclusion, that TBT is not only a catalyst but a reagent too in the studied reaction is proved experimentally. When carrying out the reaction at 150 °С with increased amount of TBT (mole ratio of DMEFDCA:TBT= l :l) the yield of target product reached 80% in 10 hours (according to NMR-spectroscopy data). However, isolation of target product was accompanied by certain difficulties, therefore another obtaining method of target product had been considered.
Another catalyst NaHSO4 [3] was used for synthesis of fluoro-containing ethers based on 2,5-furancarboxylic acid (Scheme 3).
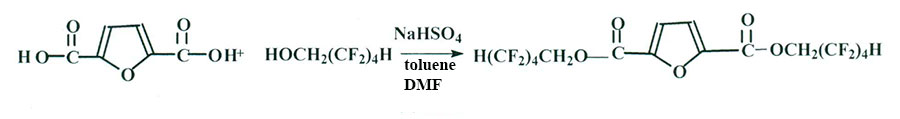
Scheme 3
The reaction proved as unrealizable even at prolong boiling over 60 hours due to light solubility of FDCA in toluene. That is why DMF was added into reaction mixture besides toluene. The reaction was carried out at 150 °С. While analyzing spectra NMR- 1 Н, 13С and 19F it was stated, that forming of fluoro-containing FDCA ester occurred, its yield was going up as reaction period increased and had reached 70% in 60 hours of boiling. Complex salt [NаНSО4 х DMF] was forming as by-product. Isolation of target product in a pure form could not have been performed.
That is why 2,5- furandicarboxylic acid dichloroanhydride [5,6] was synthesized in advance (Scheme 4) and at its next interaction with 2,2,3,3,4,4,5,5-octafluoropentan-1-ol di-2,2,3,3,4,4,5,5-octafluoropentyl 2,5-furandicarboxylate – powder-like product, well-soluble in methanol, acetone, R-113 and hexafluorobenzene was synthesized with yield of 80%. M.P. = 45-47 °С, (Scheme 5):
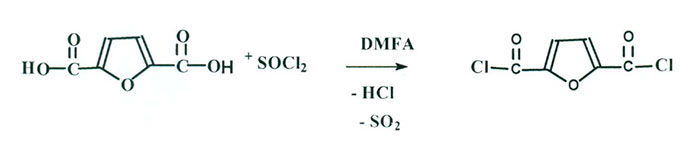
Scheme 4
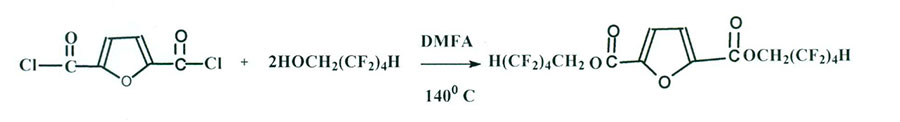
Scheme 5
IR-spectra, υ, cm-1 : 3433 (СН of furan ring) ; 1748 (С=О ester); 1134-1114 (CF2).
NMR- 1Н spectra (solution in C6F6), δ, ppm: 8.0 (s, 2Н of furan ring); 7.29-(t. CHCF2).
NMR- 19F spectra (solution in C6F6), δ, ppm: -139.76 ÷ -140 .0 (d . CF2H); -131 .5 (s. CF2CF2H); -126 .6 (s, CF2CF2CF2H); -121. 1 (s, OCH 2CF2).
Authors would like to express their gratitude to Russian Science Foundation for financial support of the current study (grant #14-50-00126).
References
- Robert Filler, Jack V.Fenner, Charles S.Stokers, Josef F. O'Brien and Murray Hauptschein. JACS (19530 75, 2693-2695. Fluorinated Esters. 111 . Diesters of Carboxylic Acids with Fluorine-containing Alcohols and Glycols. DOI: 10.1021/ja01107а042.
- Bernardm М., Woolf С. US 3438946 а 19690415. Fluoroalkyl-substitution esters, diesters, and polymers therefrom.
- Shanmugam Thiyagarajan, Aliaksei Puki n, Jacco van Haveгen, Martin Lutz, Dаап S. vаn Es. RSC Adv. 2013, 3 ,15678-15686. Concurrent formation of furan-2,5- and furan-2,4-dicardoxylic acid: unexpected aspects of the Henkel reaction .
- Kichura D.B., Yugach O.V. Khimicheskaya promyshlennost', 7, 1975, 19-21. Eterifikaciya ftalevogo angidrida spirtami, ispol'zuya titansoderzhashchie katalizatory.
- Zhang, Rong; Song Huaijun; Wu, Guowang ; Jiang, Liping; Zeng, Xiaouan ; Huang, Zhuowang; Ding, Jianzhong . Faming Zhuanli Shenqing (2013), CN l 03469348 А 20131225. Fluorinated polybutylene terephthalate drawn textured уаrn fiber and its preparation method.
- С. Morales-Huerta, Juan; М. De Harduya, Antxon; Polymer (UK) 2016, vol. 87, р. 148-158.
Recommended for publication by Prof. S.M Igumnov
Fluorine Notes, 2017, 113, 7-8
