Received: July 2017
DOI 10.17677/fn20714807.2017.04.01
Fluorine Notes, 2017, 113, 1-2
Synthesis of 3-Indolyl Perfluoroalkyl Carbinols by the Reaction of Indoles with Perfluorinated Aldehydes
D. V. Gusev, E. V. Belyaeva, A. L. Sigan, and N. D. Chkanikov
Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, Moscow, 119991 Russia
e-mail: faftor.belyaeva@mail.ru
Abstract: Perfluoroaliphatic aldehydes with different carbon chain length or their hemiacetals have been involved into selective non-catalytic hydroxyalkylation of indoles to produce the corresponding 3-indolyl perfluoroalkyl carbinols.
Key words: perfluoroaliphatic aldehydes, hydroxyalkylation, indoles, 3-indolyl carbinols
3-Indolyl perfluoroalkyl carbinols with perfluoroaliphatic fragment (RF) of different structure are of practical importance as precursors for preparing different biologically active compounds [1–4].
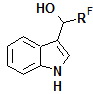
3-Indolyl carbinols with RF = CF3 are the most frequent in the literature, while carbinols with RF = C2F5 are known to a much lesser extent. Among the known methods of synthesis for these compounds, one should emphasize the reduction of 3-acylindoles [5–7], addition of Ruppert's reagent to indole-3-carboxaldehyde and its derivatives [8, 9], addition of perfluoroaliphatic aldehydes and their derivatives to indole under the action of different catalysts with opportunity to obtain of enantimeric pure 3-indolyl carbinols [10–12], and hydroxylation of indoles with polyfluoroalkanols in the presence of radical initiators [13]. Several papers reported non-catalytic reaction of trifluoroacetaldehyde with indole at ambient temperature [14] or upon heating [15]. There are no examples in the literature for the reactions of indoles and aldehydes with long-chain perfluorocarbon fragments; therefore, the study of these reactions extends the possibility of synthetic use of perfluoroaliphatic carbonyl compounds.
In this work, we have shown that the reaction of indole or N-methylindole with perfluoroaliphatic aldehydes under an inert atmosphere in the absence of any catalysts leads to formation of the corresponding 3-indolyl carbinols (1а–с and 3а–с) in 41–69% yields. Perfluoroaliphatic aldehydes, especially with short perfluorocarbon chain, are known to be volatile hygroscopic and light-sensitive compounds; therefore, the reaction was conducted in the dark under an inert atmosphere at reduced temperature. Furthermore, the careful selection of solvent allowed the maximal simplification of reaction product isolation. Thus, on the use of methylene chloride–hexane mixtures in different ratios (from 1 : 1 to 3 : 1), 3-substituted derivatives commonly precipitated from reaction mixture and required no additional purification.
It should be noted that the reaction of perfluoropropanal with unsubstituted indole led to 3-indolyl carbinol 1a as the main product along with compound 2a as a byproduct resulting from the condensation of second indole molecule. In synthesis of 1b,c the same byproducts wasn't obtained. This phenomenon can be explained by the fact that 1b,c precipitate from reaction mixture and 1a remains in solution increases the probability of it's reaction with second indole molecule.
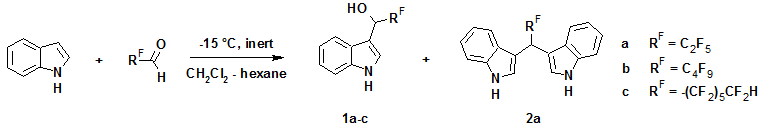
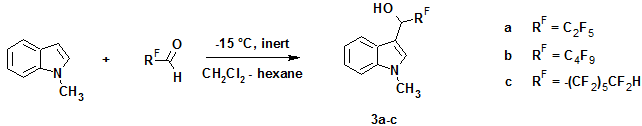
In its turn, the reaction of N-methylindole with appropriate aldehydes gave rise to formation of only 3-indolyl carbinols (3a–c) in good yields (53–65%), which considerably decreased when the reactions were carried out in air.
As already noted, the use of perfluoroaliphatic aldehydes, especially short-chain reagents, requires special conditions to store chemicals and conduct reaction. Therefore, we examined the possibility to use more stable perfluoropropanal methyl hemiacetal in the studied reactions under the same conditions. It was found that the reaction resulting in compound 1a (according to TLC data) proceeded under standard conditions at low temperature (–15оC, methylene chloride–hexane = 3 : 1, 3 days) very slowly but afforded no byproducts.
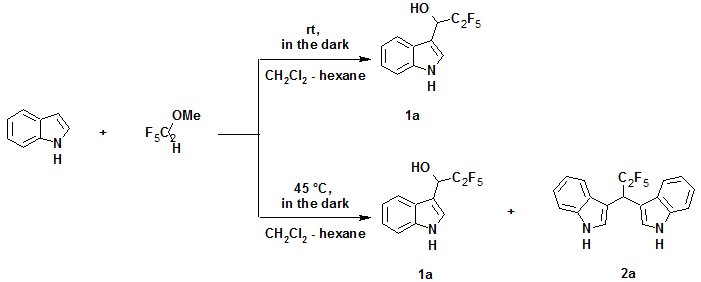
At the same time, reaction rate increased upon warming to ambient temperature, a marked amount of unreacted indole remained, the yield of 1a rose to 15%. It can be explained by presents of methanol, formed from hemiacetal during the reaction. An finally, complete indole conversion was achieved upon heating of a mixture of indole with the hemiacetal in 1 : 2 ratio up to 45°C in a glass autoclave in the dark for 30 h to give a mixture of compounds 1a and 2a in 2 : 3 ratio (total yield ~70%). The considerable growth of the share of compound 2a in this case seems to be caused by increase in reaction temperature and by the fact that product 1a did not precipitate and therefore it could more probably react with second indole molecule.
Thus, in this work, we have shown the fundamental possibility of non-catalytic selective hydroxyalkylation of indoles with the use of perfluoroaldehydes under mild conditions; the obtained 3-indolyl perfluoroalkyl carbinols can be used in the synthesis of biologically active compounds.
Experimental
NMR spectra 1H and 19F were recorded on a Bruker AMX-400 and AMX-300 spectrometer with frequency 400.13 and 376.50 MHz at 20°C. Chemical shifts of 1H and 19F were determined relatively to proton signal of solvent (dmso-d6) and trifluoroacetic acid (TFA) as external standard.
Mass-spectra (EI-DIP) were recorded on mass-spectrometer Finnigan Polaris Q, ionization energy – 70 eV, procedure of sample injection – DIP.
Monitoring of the reaction course and purity of products were controlled by Merck Kieselgel 60 F254 plates. For column chromatography silica gel (MN Kieselgel 60) and aluminium oxide (neutral, 100-200 μm) were used.
Reagents and solvents: dichloromethane (DCM was dried under CaH2 and distilled. Indole was purified by recrystallization from hexane, N-methylindole was distilled before use. Fluorinated aldehydes were distilled under conc. H2SO4 before use. Perfluoroaldehyde hydrates and perfluoropropanal methyl hemiacetal were purchased from “P&M Invest”.
Reaction of indole and N-methylindole with aldehydes
Synthesis of 2,2,3,3,3-pentafluoro-1-(1H-indol-3-yl)propan-1-ol, 1a
Typical procedure
To a cooled solution of indole (1M in DCM) 1.0 ml solution of perfluoropropanal (1M in mixture of DCM-hexane 1:1) was added under argon and kept 6 days under -15 оС. Monitoring of the reaction was controlled by TLC (silica gel, DCM). Then mixture of 1a and byproduct 3,3'-(2,2,3,3,3-pentafluoropropane-1,1-diyl)bis(1H-indole) (2a) was separate by column chromatography (silica gel, DCM). After removing of solvent 0.11 g 1a was obtained as yellow viscous oil, yield 41%. 1Н NMR (dmso-d6, , ppm): 5.46 (dt, 1H, J1 = 20.35 Hz, J2 = 5.81 Hz, CH(OH)); 6.55 (d, 1H, J = 5.09 Hz, CH(OH)); 7.04 (t, 1H, J = 7.31 Hz, Ind-H); 7.12 (t, 1H, J = 7.31 Hz, Ind-H); 7.42 (d, 1H, J = 8.27 Hz, Ind-H); 7.50 (d, 1H, J = 2.54 Hz, Ind-H); 7.68 (d, 1H, J = 7.95 Hz, Ind-H); 11.28 (brs, 1H, NH). 19F NMR (dmso-d6, , ppm): -49.97 (d, 1F, J = 268.55 Hz, part А of АВ-system, CF2); -40.85 (d, 1F, J = 268.55 Hz, part B of АВ-system, CF2); -2.02 (s, 3F, CF3).
Compound 2a was obtained as yellow oil. 1Н NMR (dmso-d6, , ppm): 5.51 (t, 1H, J = 19.17 Hz, CH(C2F5)); 7.01 (t, 2H, J = 7.32 Hz, Ind-H); 7.08 (t, 2H, J = 7.32 Hz, Ind-H); 7.36 (d, 2H, J = 8.04 Hz, Ind-H); 7.58 (d, 2H, J = 2.03 Hz, Ind-H); 7.74 (d, 2H, J = 7.83 Hz, Ind-H); 11.18 (brs, 1H, NH). 19F NMR (dmso-d6, , ppm): -36.12 (s, 2F, CF2); -1.97 (s, 3F, CF3). MS (EI-DIP), m/z: 121.9, 218.1, 245.0, 363.9.
Compounds 1b,с и 3а-с were obtained by the same way. Reaction time varied from 1 day (for 1b and 3b-c) to 4 or 6 days for 1с и 3а respectively. Compounds 1b,с and 3а-с are white or light-yellow solid and precipitate from reaction mixture.
1b (2,2,3,3,4,4,5,5,5-nonafluoro-1-(1H-indol-3-yl)pentan-1-ol): reaction time – 1 day, 0.25 g of 1b was obtained as uncolored crystals, yield 69%. 1Н NMR (dmso-d6, , ppm): 5.53 (dt, 1H, J1 = 21.36 Hz, J2 = 5.85 Hz, CH(OH)); 6.54 (d, 1H, J = 4.83 Hz, CH(OH)); 7.04 (t, 1H, J = 7.12 Hz, Ind-H); 7.12 (t, 1H, J = 7.12 Hz, Ind-H); 7.42 (d, 1H, J = 7.88 Hz, Ind-H); 7.50 (d, 1H, J = 2.29 Hz, Ind-H); 7.67 (d, 1H, J = 7.88 Hz, Ind-H); 11.28 (s, 1H, NH). 19F NMR (dmso-d6, , ppm): -47.29 (m, 3F, CF2CF2); -44.57 (d, 1F, J = 296.2 Hz, part А of АВ-system, CF2); -42.74 (d, 1F, J = 296.2 Hz, part B of АВ-system, CF2); -38.03 (d, 1F, J = 275.25 Hz, part А of АВ-system, CF2); -2.28 (s, 3F, CF3). MS (EI-DIP), m/z: 69.1; 91,1; 118.0; 131.0; 145.9; 150.9; 177.9; 328.0; 346.9; 364.9; 393.9.
1c (2,2,3,3,4,4,5,5,6,6,7,7-dodecafluoro-1-(1H-indol-3-yl)heptan-1-ol): reaction time – 4 day, 0.2 g 1c was obtained as, yield 45%. 1Н NMR (dmso-d6, , ppm): 5.51 (d, 1H, J = 21.00 Hz, CH(OH)); 6.50 (d, 1H, J = 4.77 Hz, CH(OH)); 6.99-7.35 (m, 3H, Ind-H and CF2H); 7.41 (d, 1H, J = 8.01 Hz, Ind-H); 7.49 (m, 1H, Ind-H); 7.66 (d, 1H, J = 7.63 Hz, Ind-H); 11.27 (s, 1H, NH). 19F NMR (dmso-d6, , ppm): -60.47 (d, 1F, J = 310.67 Hz, part А of АВ-system, CF2H); -59.97 (d, 1F, J = 310.67 Hz, part B of АВ-system, CF2H); -50.95 (s, 2F, CF2CF2H); -45.87 (d, 1F, J = 274.93 Hz, part А of АВ-system, CF2C(OH)); -44.93 (d, 2F, J = 145.71 Hz, CF2); -43.76 (d, 2F, J = 118.22 Hz, CF2); -42.69 (d, 2F, J = 129.22 Hz, CF2); -37.91 (d, 1F, J = 274.93 Hz, part B of АВ-system, CF2C(OH)). MS (EI-DIP), m/z: 91.1; 117.0; 118.0; 145.9; 150.9; 177.9; 189.9; 428.8; 446.7.
3a (2,2,3,3,3-pentafluoro-1-(1-methyl-indol-3-yl)propan-1-ol): reaction time – 6 days, 0.18 g of 3a was obtained as white solid, yield 65%. 1Н NMR (dmso-d6, , ppm): 3.81 (s, 3H, CH3); 5.46 (dt, 1H, J1 = 20.60 Hz, J2 = 6.10 Hz,CH(OH)); 6.58 (d, 1H, J = 5.34 Hz, CH(OH)); 7.08 (t, 1H, J = 7.88 Hz, Ind-H); 7.19 (t, 1H, J = 7.88 Hz, Ind-H); 7.45 (d, 1H, J = 8.39 Hz, Ind-H); 7.49 (m, 1H, Ind-H); 7.68 (d, 1H, J = 7.88 Hz, Ind-H). 19F NMR (dmso-d6, , ppm): -50.13 (d, 1F, J = 268.55 Hz, CF2); -40.72 (d, 1F, J = 268.55 Hz, CF2); -1.98 (s, 3F, CF3). MS (EI-DIP), m/z: 117.0; 132.0; 159.9; 278.8.
3b (2,2,3,3,4,4,5,5,5-nonafluoro-1-(1-methyl-indol-3-yl)pentan-1-ol): reaction time – 1 day, 0.2 g 3b was obtained as light-yellow solid, yield 53%. 1Н NMR (dmso-d6, , ppm): 3.81 (s, 3H, CH3); 5.52 (d, 1H, J = 21.11 Hz, CH(OH)); 6.57 (s, 1H, CH(OH)); 7.07 (t, 1H, J = 7.51 Hz, Ind-H); 7.19 (t, 1H, J = 7.50 Hz, Ind-H); 7.45 (d, 1H, J = 8.39 Hz, Ind-H); 7.50 (s, 1H, Ind-H); 7.67 (d, 1H, J = 7.63 Hz, Ind-H). 19F NMR (dmso-d6, , ppm): -51.12 – -48.28 (m, 3F, CF2CF2); -46.93 (d, 1F, J = 296.02 Hz, part А of АВ-system, CF2); -45.19 (d, 1F, J = 296.02 Hz, part B of АВ-system, CF2); -40.43 (d, 1F, J = 277.71 Hz, part А of АВ-system, CF2); -4.70 (s, 3F, CF3). MS (EI-DIP), m/z: 117.0; 132.0; 159.9; 223.9; 343.0; 378.8.
3c (2,2,3,3,4,4,5,5,6,6,7,7-dodecafluoro-1-(1-methyl-indol-3-yl)heptan-1-ol): reaction time – 1 day, 0.27 g of 3c was obtained as white solid, yield 59%. 1Н NMR (dmso-d6, , ppm): 3.80 (s, 3H, CH3); 5.51 (d, 1H, J = 20.35 Hz, CH(OH)); 6.54 (s, 1H, CH(OH)); 6.97-7.35 (m, 3H, Ind-H and CF2H); 7.45 (d, 1H, J = 8.27 Hz, Ind-H); 7.50 (s, 1H, Ind-H); 7.67 (d, 1H, J = 7.63 Hz, Ind-H). 19F NMR (dmso-d6, , ppm): -60.47 (d, 1F, J = 312.81 Hz, part А of АВ-system, CF2H); -59.95 (d, 1F, J = 312.81 Hz, part B of АВ-system, CF2H); -50.92 (s, 2F, CF2CF2H); -45.95 (d, 1F, J = 276.19 Hz, А часть АВ-системы, CF2C(OH)); -44.91 (d, 2F, J = 146.48 Hz, CF2); -43.75 (d, 2F, J = 122.07 Hz, CF2); -42.65 (d, 2F, J = 128.17 Hz, CF2); -37.83 (d, 1F, J = 276.19 Hz, part B of АВ-system, CF2C(OH)). MS (EI-DIP), m/z: 116.9; 132.0; 159.9; 160.9; 460.8.
Reaction of indole with perfluoropropanal methyl hemiacetal
Synthesis of 2,2,3,3,3-pentafluoro-1-(1H-indol-3-yl)propan-1-ol, 1a
To 1 ml of indole solution (1M in DCM) 1 ml of perfluoropropanal methyl hemiacetal solution (1M in mixture of DCM and hexane 1:1) was added under argon, and kept under rt with protection from light for 4 days. Monitoring of the reaction course was made by TLC (silica gel, DCM). After purifying by column chromatography 0.04 g of compound 1a was obtained (yield 15%), and significant quantity of unreacted indole was remained.
If the reaction carried out in glass autoclave at the ratio of indole:hemiacetal = 1:2 and heating to 45˚С, compound 1a will be obtained in yield 45%. The other reaction conditions - reagents concentration and solvent - were the same, the reaction time was 30 hours. But the byproduct 2a was also obtained in this conditions in yield 49% relative to indole.
References
- Katayama, M.; Gautam, R. K. Biosci. Biotechnol. Biochem. 1996, 60 (5), 755–759.
- Török, B.; Sood, A.; Bag, S.; Kulkarni, A.; Borkin, D.; Lawler, E.; Dasgupta, S.; Landge, S.; Abid, M.; Zhou, W.; Foster, M.; Levine, H.; Török, M. ChemMedChem 2012, 7 (5), 910–919.
- Satoh, A.; Nishina, Y. Cell-death inhibitor, and novel compound containing indole compound or benzoxazole compound, and preparation thereof. WO2014136808 A1, September 12, 2014
- Sayed, M. T. El; Ahmed, K. M.; Mahmoud, K.; Hilgeroth, A.; El Sayed, M. T.; Ahmed, K. M.; Mahmoud, K.; Hilgeroth, A. Eur. J. Med. Chem. 2015, 90, 845–859.
- Yao, S. J.; Ren, Z. H.; Wang, Y. Y.; Guan, Z. H. J. Org. Chem. 2016, 81 (10), 4226–4234.
- Vitaku, E.; Smith, D. T.; Njardarson, J. T. Angew. Chemie - Int. Ed. 2016, 55 (6), 2243–2247.
- Kucher, O. V.; Kolodiazhnaya, A. O.; Smolii, O. B.; Prisuazhnyk, D. V.; Tolmacheva, K. A.; Zaporozhets, O. A.; Moroz, Y. S.; Mykhailiuk, P. K.; Tolmachev, A. A. European J. Org. Chem. 2014, 2014 (34), 7692–7698.
- Dong, J.; Pan, L.; Xu, X.; Liu, Q. Chem. Commun. 2014, 50 (94), 14797–14800.
- Kosobokov, M. D.; Levin, V. V; Struchkova, M. I.; Dilman, A. D. Org. Lett. 2014, 16 (14), 3784–3787.
- Borkin, D. A.; Landge, S. M.; Török, B. Chirality 2011, 23 (8), 612–616.
- Gong, Y.; Kato, K. J. Fluor. Chem. 2001, 108 (1), 83–86.
- Mitchell, L.; Williamson, P.; Ehrlichov, B.; Anderson, A. E.; Seymour, V. R.; Ashbrook, S. E.; Acerbi, N.; Daniels, L. M.; Walton, R. I.; Clarke, M. L.; Wright, P. A. Chem. - A Eur. J. 2014, 20 (51), 17185–17197.
- Xu, Z.; Hang, Z.; Chai, L.; Liu, Z.-Q. Org. Lett. 2016, 18 (18), 4662–4665.
- Landge, S. M.; Borkin, D. A.; Török, B. Tetrahedron Lett. 2007, 48 (36), 6372–6376.
- Maki, Y.; Kimoto, H.; Fujii, S.; Senga, M.; Cohen, L. A. J. Fluor. Chem. 1988, 39 (1), 47–59.
Recommended for publication by Prof. N. D. Chkanikov
Fluorine Notes, 2017, 113, 1-2
