Received: June 2017
DOI 10.17677/fn20714807.2017.03.02
Fluorine Notes, 2017, 112, 3-4
OXIDATION OF DERIVATIVES OF POLYFLUOROALKYL ESTERS OF RESIN ACIDS
L. M. Popova1, J. L. Matveenko1, S. V. Vershilov2
1Saint Petersburg State University of Industrial Technologies and Design,
Ivan Chernykh str., 4, Saint Petersburg, 198095, Russia,
е-mail: lorapopova@mail.ru
2 Federal State Unitary Enterprise S.V. Lebedev Institute of synthetic rubber, Hapsalskaya str. 1, Sankt-Peterburg, Russia, 198035,
Annotation: Oxidation of abietic acid and its polyfluoroalkyl esters using hydrogen peroxide over FeSO4 results in formation of mixture of 7-oxi, 7,15-dioxi- and 7-oxoderivatives of dehydroabietic acid.
Key words: oxidation; polyfluoroalkyl ester of resin acids.
INTRODUCTION
A significant number of researches is devoted to studying the oxidation processes of abietane and pimarane type-diterpenes [1-3]. The sensitivity of resin acids to heating, influence of oxygen and light, mineral acid is due to the presence of unsaturated bonds system. The acids of abietane row are inclined to valent isomerism as well as to disproportioning (intramolecular movement of hydrogen atoms). Thus, the abietic acid is isomerized into dehydroabietic acid under the influence of catalyst and at heating. A wide range of oxidation products among which are endo- and hydroperoxides, epoxides, hydroxy- and keto- derivatives is another consequence of hydrogen atoms high lability in structures of tricyclic diterpenoids.
The products of oxidation of tricyclic diterpenoids derivatives are of interest due to their wide range of biological activity [3]. Using of some derivatives of tricyclic diterpenic acids (modified resin) in different fields of technics, for example, as isolation materials in electrotechnics [4], also gives grounds for studying the oxidation processes of those products. The introduction of hydrophobic fluorinated substituent can significantly improve the operational characteristics of materials.
Oxidation products of abietic acid and its esters with some polyfluoroalkanols have been studied under the influence of Н2О2 (30%-solution) over Fe2SO4 as catalyst in present work. The establishing of main directions of oxidation was of our interest.
EXPERIMENTAL
NMR 1Н and 19F spectra were recorded on Bruker-500 at 500 MHz (470 MHz – for 19F) and Bruker Avance III-400 at 400 MHz (370 MHz – for 19F) in CDCl3 solution, external reference – CCl3F.
IR spectra were recorded using Shimadzu IR Prestige-2, KBr glass (thin layer, solution in CCl4) and Shimadzu FTIR-8400S (KBr tablets, thin film).
UV-spectra were recorded using SF-2000 spectrophotometer, ethanol (96%) at c 10-4 mole/l, thickness of absorbing layer is 1 cm.
The control over reactions passing process was carried out using TLC method at Sorbfil plates, eluent: hexane–methylenechloride – acetone (1–1–0,5).
Abietic acid (1) was isolated from SYLVAROS®85 Tall Oil Rosin (softening point is 63°С, Acid Number 168, produced by Arizona Chemical) using methods [5]. Esters of abietic acid were obtained using esterification reaction of abietic acid with corresponding polyfluorinated alcohols: trifluoroethanol (2), 1Н,1Н,3Н-trihydrotetrafluoropropanol (3), 1Н,1Н,5Н-trihydro-octafluoropenanol (4) [6].
Oxidation of abietic acid (1). 0,5 g (1.65 mmol) of abietic acid (1) was dissolved in 11 ml of toluene in flat-bottom flask equipped with a reflux condenser, 0.2 g (5.88mmol) of Н2О2 (30%-solution) and 0.005 g (0.03 mmol) of FeSO4 were added. The reaction mixture was kept at 30°С and intensive mixing for 5 hours. The reaction mixture was extracted using diethyl ether and washed by water till reaching neutral reaction, ether solution was dried using Na2SO4 (calcined), drying agent was filtered, solvent was distilled and the residuum was kept in vacuum over Р2О5. The yield of oxidation products is 0.35 g (70%), yellow oil, Rf 0.65. IR-spectra (film), cm-1: 3517 (νOH), 3005, 2959, 2930, 2870, 2653, 2535 (νCH), 1730, 1694 (νCO). UV-specrum (EtOH), nm (lg ε): 205 (4.44), 240 (4.57), 270 (shoulder). NMR 1Н spectra, δ, ppm: 5.40 (s. 1Н, С7Н), 5.80 (s. 1Н, С14Н), 6.91 (s. 1H, Car14H), 7.02 (d. 1H, Car12H), 7.16 (d. 1H, Car11H), 7.20 (d. 1Н, С11αН), 7.31 (d.d. 1Н, С12αН), 7.54 (d. 1Н, С14Н), 7.91 (s. 1H, Car14H), 8.02.
Oxidation of trifluoroethylabietate (2). It was done in analogous manner (1), after processing of solution of 0.57 g (1.47 mmol) of trifluoroethylabietate (2) in 6 ml of toluene 0.2 g (5.9 mmol) Н2О2 (30%-solution), 0.0056 g (0.032 mmol) FeSO4, 0,38 g (66%) obtained, MP is 86-87°С, Rf 0.31. IR- spectra (film), cm-1: 3688, 3597, 3510 (νOH), 3027, 3014, 2934, 2873, 2654 (νCH), 1738, 1697 (νCO), 1285, 1229, 1173 (νCF). UV-spectra (EtOH), nm (lg ε): 211 (3.25). 244 (shoulder). NMR 1Н, δ, ppm. 0.86 (3Н, C20H3), 1.01 and 1.02 (6Н, C16,17H3), 1.26 (3Н, C19H3), 2.24 (1Н, C15H), 2.35, 2.39, 2.86, 4.48 (m. 2Н, СН2О, J=8.5 Гц), 6.91 (s. 1H, Сar14H), 7.02 (d. 1H, Сar12H); 7.16 (d. 1H,11H), 7.32 (d.d. 1Н, С12αН), 7.44 (d. 1Н, С14Н), 7.51 (d. 1Н, С14Н), 7.76, 7.90 (s. 1H, Car14H), 8.10. NMR 19F, δ, ppm.: - 73.65 (d. 3F, CF3, J=50 Hz).
Oxidation of 1Н,1Н,3Н-trihydrotetrafluoropropylabietate (3). Oxidation of 0.38 g (0.9 mmol) of 1Н,1Н,3Н-trihydrooctafluoropropylabietate in 10 ml of toluene was carried out in analogous manner (1) by processing of 0.1 g (2.9 mmol) Н2О2 (30%-solution) over 0.0038 g (0.025 mmol) FeSO4. Yield (3) 0.18 g (45 %), liquid amorphous substance. IR-spectra (film), cm-1: 3664, 3597, 3512 (νOH), 3027, 3010, 2933, 2872, 2652, 2537 (νCH), 1696 (νCO), 1190, 1152, 1124, 1111 (νCF). UV-spectra (EtOH), nm (lg ε): 204 (4.36), 240 (shoulder), 252 (3.85), 270 shoulder. NMR 1Н spectra δ, ppm: 4.32 (t. 2Н, ОСН2, J=12.8 Hz), 5.39 (s. 1Н, С7Н), 5.73 (1Н, С14Н), 5.79 (s. 1Н, С14Н), 5.83 (t.t. 2Н, CF2H, J′=53.4 Hz), 6.90 (s. 1Н, Сar14Н), 7.00 (d. 1Н, Сар12Н), 7.16 (d. 1Н, Сar11Н), 7.33 (d.d. 1Н, С12αН), 7.38, 7.44 (d. 1Н, С14Н), 7.54 (d. 1Н, С14Н), 7.89 (s. 1H, Car14H), 8.09. NMR 19F spectra, ppm: –133.96 (d. 2F, C3F2H, J=53.4 Hz), –125.84 (t. 2F, C2F2).
Oxidation of 1Н,1Н,5Н-trihydrooctafluoropentylabietate (4) Oxidation of 0.75 g (1.6 mmol) 1Н,1Н,5Н-trihydrooctafluoropentyl-dehydroabietate (4) in 15 ml of toluene was carried out in analogous manner (1) 0.2 g (5.88 mmol) of Н2О2 (30%-solution) over 0.0075 g (0.049 mmol) FeSO4. Yield 0.63 g (84%), umber oil, Rf 0.66. IR-spectra (film), cm-1: 3671, 3603, 3517 (νOH), 3027, 3003, 2957, 2932, 2870, 2660 (νCH), 1738, 1695 (νCO), 1261, 1170, 1119, 1114 (νCF). UV-spectra (EtOH), nm (lg ε): 208 (4.11), 218 (3.72), 270 (shoulder). NMR 1Н spectra ppm.: 4.51 (t. 2Н, OCH2 J=20 Hz,), 5.88 (t.t. 1Н, CF2H J'=52, J''=4.5 Hz), 6.92 (s. 1H, Сar14H), 7.04 (d. 1H, Сar12H), 7.19 (d. 1H, С11H), 7.31 (d.d. 1Н, С12αН), 7.43 (d. 1Н, С14Н), 7.90 (s. 1H, Car14H). NMR 19F, δ, ppm.: –136 (d. 2F, CF2H), -129 (2F, C4F2), -124 (2F, C2F2), -118 (2F, C3F2).
RESULTS AND DISCUSSON
Depending on activity of oxidation system the following directions of oxidation of abietic acid (its esters) are possible: “C” ring aromatization, formation of oxy- and oxa-derivatives, opening up of “C” ring.
When KMnO4 is acting the abietic acid and its esters form 13,14-diols and 7,8,13,14-tetrols in consistent manner [3]. The processing using such reagents as SeO2 (C6H6) [7, 8] and Hg(OAc)2 [3] results in “C” ring aromatization as well as in appearing of ОН-functions at С-7. Forming of 13,14-epoxide and 7,8,13,14-diepoxide occurs when acetic hydroperoxide is used [9, 10]. The mixture of 13-epoxy-, 13,14-dihydroxy- and 7,8,13,14-tetrahydroxy derivatives forms as a result of abietic acid methyl ester oxidation using monoperphthalic acid [11]. The mixture of 12-keto-, 6,12-diketoabietates and 7-ketodehydroabietate was obtained when using CrO3 – HOAc (0°C) system [3]. The disclosing of “C” ring occurs when using Pb(OAc)4, as well as during ozonolysis [3].
The presence of dehydroabietic, 7-hydroxidehydroabietc and 7-dicarbonyldehydroabietic acid was established among main products of abietic acid Н2О2 oxidation over catalytic complex: [π-C5H5N(CH2)15CH3]3PW12O40 [12].
Oxidation of abietic acid (1) as well as its esters - trifluoroethylabietate (2), 1Н,1Н,3Н-trihydrotetrafluoropropylabietate (3), 1Н,1Н,5Н-trihydrooctafluoropentyldehydroabietate (4) by triple excess (mole) of 30% solution of Н2О2 over Fe2SO4 in toluene for 5 hours (see scheme).
Scheme
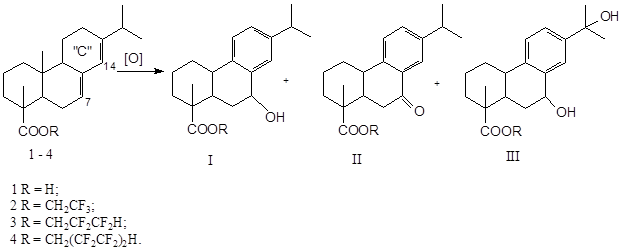
For IR-spectra of oxidation products the stripes of valent oscillations of C-H links in the field of 3027-2535 cm-1 are typical as well as oscillations of carbonyl group of C=O at 1738-1730 and 1694-1696 cm-1. Intensive signals in the interval of 1285-1037 cm-1 сcorrespond to νC-F valent oscillations in the spectra of oxidation products of 2-4 compounds. Besides that, in the spectra of all oxidized compounds new stripes of valent oscillations of hydroxyl groups are appearing in the field of 3688-3510 cm-1.
The considering of protons signals field at С7 and С14, as well as at С11 and С12 (Table) provides certain information on composition of compounds 1-4 oxidation products. The presence of pair of δ 5.36 – 5.40 and 5.76 – 5.80 ppm signals corresponding to vinyl protons of –С7=С8–С14=С13– fragment [13] is typical for derivatives of abietic acid (1-4). In spectra of oxidation products this pair is also present in case of compounds 1 (5.40 and 5.80) and 3 (5.36 and 5.78 ppm.). The signals of protons of aromatic ring are appearing in several series (I – III) depending on disposition and quantity of hydroxyl or carbonyl functions within the interval of 6.9 – 7.9 ppm (Table) [14, 15, 13].
Table
|
Position in Molecule |
Position of Protons Signals in Compounds Spectra (δ, ppm): |
|||
|
DHAC* |
I |
II |
III |
|
|
Н-11 |
7.13 |
7.16 d (8.1) |
7.30 d (8.1) |
7.20 d (8.5) |
|
H-12 |
7.00 |
7.09 dd (1.9 and 8.2) |
7.41 dd (8.4 and 2.2) |
7.31 dd (8.5 and 1.6) |
|
H-14 |
6.88 |
7.38 д (1.8) |
7.78 д (2.4) |
7.44 д (1.6) |
|
Sources |
[13] |
[15] |
[15] |
[14] |
* dihydroabietic acid
Comparing experimental and literature data we make a conclusion, that obtained oxidation products of compounds 1-4 represent mixtures of hydroxy- and oxy- derivatives mainly of aromatic row. Series of signals of δ (ppm.) 7.16, 7.00–7.02 and 6.90–6.92 (С-11, С-12, С-14) corresponds to aromatic fragment of dehydroabietic acid and its esters. 7,15-dihydroxiderivatives (III) are among oxidation products: series of signals of 7.18–7.20 (d), 7.28–7.30 (m) and 7.43–7.44 (d). The signals of small intensity of δ 7.16 (d) ppm point out in presence of 7-hydrooxiderivatives (I) in the oxidation products of compounds 1-3 and signals of δ 7.89–7.91 ppm the presence of 7-oxoderivatives (II) in oxidation products of compounds 1, 3, 4.
In 1H NMR spectra of compounds (2-4) the signals of protons of –ОСН2– group of polyfluoroalkyl fragment are present in the form of multiplets with chemical shifts of 4.48 ppm (2) and 4.32 ppm (3), 4.51 ppm (4). The signals of methane protons of –CF2H ending groups are appearing in the form of triplet of triplets with chemical shifts of 5.83 ppm (3), 5.88 ppm (4).
In UV spectra of oxidation products the absorption maximums are present in the field of 205-218 and 240-250 nm, the absorption of aromatic system is appearing in the form of shoulder in the field of 270 nm.
CONCLUSION
The oxidation of abietic acid and its esters with some polyfluoroalkanols results in aromatization of “C” ring and formation of compounds containing hydroxyl groups in positions C-7 and (or) C-15 as well as carbonyl group in position of C-7 under the influence of Н2О2 (30%-solution) at catalysis of FeSO4 (30°С, 5 h).
REFERENCES
- Simonsen J. The terpenes. V. III. The sesquiterpenes, diterpenes and derivatives. Cambridge University press, 1952. 579 p.
- Zandermann W/ NaturHze Terpentinoltallol (Chemie und technologie). Berlin (Gottingen) Heidelberg. Springer Verlag. 1960.
- Tolstikov G.A., Tolstikova T.G., Shultz E.E., Tolstikov S.E., Khvostov E.E. Resin acids from Russian forest conifers. Chemistry and pharmacology. Ed. By G.A.Tolstikov. Novosibirsk. Acad. Publishing House “Geo”. 2011. 396 с.
- RU 2263360 (C1). Cl H01B3/20; H01B3/42. Cable impregnating compound./ Barsukov E.V., Barsukov V.K., Demin A.V., Kurashov D.A. appl. 19.05.2004. publ. 27.10.2005.
- Lazur’evskii G.V., Terent’eva I.V., Shamshurin A.A. Practical works in chemistry of natural compounds. Iss. I. Мoskow. Vysshaya shkola. 1961. P. 102-103.
- Nyanikova G.G, Popova L.M., Gaidukov I.N., Shabrina O.P., Vershilov S.V. Russ. J. Gen. Chem. 2013. V. 83. No 13. P. 2738.
- Esqudero J., Marques C., Rabanal R.M., Valverde S. Tetrahedron. 1983. V. 39. P. 3167.
- Suryawashi S.N., Rani A., Dhami T.S., Bhakuni D.C. Synth. Commun. 1989. V. 19. N. 17. P. 2927.
- Arbuzov B.A., Hismatullina А.G. Izv. Аkad. nauk USSR, ser. khim. 1961. № 7. P. 1280.
- Silko Y.A., Raldugin V.А., Shchmidt E.N., Мамаtiyк V.I., Pentegova V.А. Izv. Sibirskogo otd. Russ. Akad. nauk USSR. Ser. khim. 1983. № 4/2. С. 124.
- Titov V.V., Popa D.P., Lazur’evskii G.V. Russ. J. Gen. Chem+. 1969. V. 38. P. 71.
- Dao-zhan H, Hong-yun L., Tao Z. Journal of Fujian Forestry Science and Technology. 2009. Р. 38.
- Brannon D.R., Boaz H., Wiley B.Z., Mabe J., Horton D.R. J. Org. Chem. 1968. V. 33. N. 12. P. 4462.
- Prinz S., Müllner U., Heilmann J., Winkelmann K., Sticher O., Haslinger E., Hufner A. J. Nat. Prod. 2002. V. 65. P. 1530.
- Monteiro S.M.C.S., Silvestre J.D., Silva A.M.S., Cavaleiro J.A.S., Felix V., Drew M.G.B. New J. of Chem.. 2001. V. 25. Р. 1091.
Recommended for publication by V. Kornilov
Fluorine Notes, 2017, 112, 3-4
