Received: December, 2016
DOI 10.17677/fn20714807.2016.06.02
Fluorine Notes, 2016, 109, 3-4
ANTIMICROBIAL PROPERTIES OF POLYDIMETHYLSILOXANE SURFACE GRAFTED WITH PERFLUOROHEXANOIC ACID
T. I. Karpunina1, D. E. Yakusheva2, I. A. Kasatkin2, L. Yu. Nesterova3, Yu. B. Busyrev3, R. M. Yakushev2
1Perm State Medical University after E. A. Wagner, Perm, Russia, 614990, e-mail: karpuninapsma@mail.ru
2Institute of Technical Chemistry, Russian Academy of Sciences, the Ural Branch, Perm, Russia, 614013, e-mail: ambersofdawn@gmail.com
3Institute of Ecology and Genetics of Microorganisms, Russian Academy of Sciences, the Ural Branch, Perm, Russia 614081, e-mail: larisa.nesterova@bk.ru
Abstract: Antimicrobial chemical modification of polydimethylsiloxane (PDMS) surface has been carried out. Bacteria biomass has been estimated within the suspension in dynamics of periodic growth rate via optical density (OD) at a wavelength λ = 600 nm. The modified samples have been shown to possess antimicrobial effect towards gram-negative and gram-positive taxons more significantly suppressing the growth of S.epidermidis. According to optical microscopy observations the prolonged microbial contact with modified PDMS samples leads to practically two times reduction of the area occupied by staphylococcus biofilm and four times reduction of the area occupied by klebsiella biofilm. The inhibitory effect has been confirmed by the results of total protein determination.
Keywords: Polydimethylsiloxane, modification, perfluorohexanoic acid, antibacterial properties, S. epidermidis, K. pneumoniae, biofilm, optical density.
Introduction
Recently polymer materials used in endoprosthesis has been under intense development. There is a definite success in this field, however, the area of surgical operation is often infected due to the ability of microorganisms to colonize both biogenic and abiogenic surfaces. In general in surgical practice acute and chronical inflammatory processes triggered by biofilm are quite common. It is connected with a wide application of implantates – catheters and drainage tubes, various prosthesis, suture materials etc. It is evident that development of the methods for polymer surface treatment aimed at suppression of biofilm formation is one of the most urgent problems of medicine [1,2]. For the microbe colonization to be prevented, the polymer surface can be modified by physical or chemical methods or their combination. The coatings preventing microbe adhesion, killing bacteria at contact or releasing antibacterial compounds are being developed [3-5].
Modification of an inert surface of silicon rubber is a difficult task. There is a number of approaches for introduction of reactive groups into the structure of the surface macromolecules. Earlier the authors used pulsed ion-beam treatment for activation of the surface followed by acrylic acid grafting and then complex-bound zinc layer onto the surface of PDMS samples. Antibacterial action of the modified materials in relation to S. еpidermidis was demonstrated [6]. Last decades fluorine-containing reagents endowing the surface layer with hydrophobic, oleophobic and antibacterial properties attracted increasing interest of the researchers. For example, in [7] antibacterial treatment of the fibers using fluoroalkylsilane coating as the first stage was suggested. The synthesis and antibacterial properties of perfluoroalkyl substituted quarternary ammonium salt was described in [8]. Besides, antimicrobe action of heterocyclic compounds with perfluorinated substitutes was shown in [9]. All this taken into account, it was considered a good idea to broaden the spectrum of antibacterial surfaces and test the PDMS samples with surface grafted perfluorinated С6 radicals. The etching by piranha solution (the mixture of sulfuric acid and hydrogen peroxide ) was chosen as a technique for surface activation. It was shown, that even a short-time exposure to piranha solution results in formation of hydroxyls on the polymer surface [10]. So, it is possible to carry out further chemical transformations of the surface layer.
The present paper is aimed at estimation of the suggested modification of PDMS surface on the growth and biofilm formation of the bacteria from different taxonomic groups.
Experimental
For preparation of PDMS samples siloxane oligomer SKTN-1, crosslinking agent tetraethoxysilane (TEOS) and dibutyltin dilaurate in the ratio 100 : 0.3 : 0.03 parts by weight were mixed. The mixture was dosed into the 12- well polystyrene plates (Orange Scientific, Belgium) – 1 ml per well. PDMS samples were vacuumed for complete removal of unreacted TEOS and low-molecular by-products. Some of the wells were surface-modified for imparting of antibacterial properties according to the scheme presented in Fig.1. First PDMS surface was activated via treatment by the solution containing 93% sulfuric acid and 30% hydrogen peroxide in the ratio 3:1. After the treatment hydroxyl groups are assumed to be introduces into the structure of the surface macromolecules. Upon multiple washing by deionized water the wells were filled by 1% solution of 3-aminopropyltriethoxysilane (grade “pure”, Fluka AG, Switzerland) in isopropanol and kept at ambient temperature for 2 hours. Then the surface was thoroughly rinsed with water and the wells were filled with 15% water solution of aminocaproic acid. The reaction was carried out in the covered plates at 60°С for 5 hours.
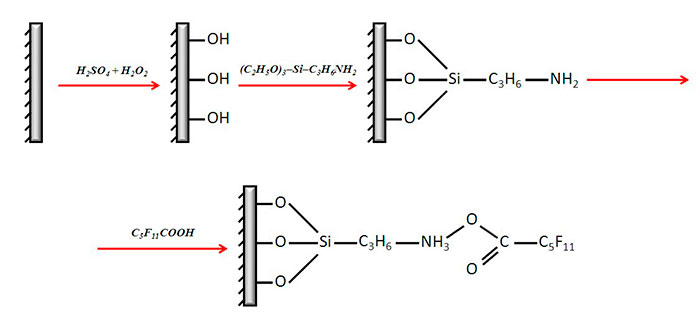
Fig. 1. The scheme of PDMS surface modification.
Bacteria-associated contamination of polymer medical devices is usually due to Firmicutes и Gracilicutes. So, typical representatives of these phyla - clinical and museum strains of Staphylococcus epidermidis and Klebsiella pneumonia – were used as test-cultures. The 24 hours broth cultures matching a 2.0 McFarland turbidity standard diluted in LB-broth in the ratio 1:100 were introduced into the plate wells (2 ml per well). Four parallel experiments were carried out in each case. The plates were covered and incubated under static conditions in a wet thermostat chamber at 37°С up to 8 days.
For comparative estimation of the growth characteristics of the cultures and biofilm formation on the modified and initial PDMS samples, a multimodal plate reader TECAN Infinite M200 (Austria) was used. The parameters were analyzed using the optical density (wavelength 600 nm) measurements. During the first 24 hour period the total biomass increase was monitored every hour. The following technique was used for estimation of the contribution of plankton and sessile components after 1, 2, 3, 6 and 8 days of the microbe strain incubation under static conditions at 37°С. The plated were subjected to shaking at 260 rpm for 2 hours, then 200 mcL aliquates of the suspended microbe culture were poured into the wells of the 96-well microtitration plates (MiniMed, Russia), then OD600 was measured by the reader. The residual plankton was removed, and the biofilms were rinsed 5 times with 2 ml portions of sterile deionized water for 5 minutes in a shaker.
Experimental OD600 values for the parallel samples were verified for the presence of rough errors using the Dixon's Q test at confidence probability 0.95. In parallel experiments for OD600 values that passed the Dixon's Q test, the mean value and standard deviation was calculated by the known formulae. The calculated mean values of OD600 were approximated by a sigmoid function. The statistical treatment, approximation and graphic presentation of the data were carried out using the table processor Microsoft Office Excel 2007 with “Data Analysis” and “Excel Neural Package” program packages.
The total protein in the biomass adhered onto the polymer surface was determined by Lowry's method after 5 sonication cycles each for 30 s on the ice bath using an Ultrasonic processor Cole Parmer (USA, 130Watt, 20kHZ)
The biofilms, formed on the surface of the samples were dyed by 0,1% aqueous solution of gentian violet . The biofilms were visualized using an Olympus light microscope BX 5. The biofilm area was calculated using an ImageScope software at an automatic highlighting regime by ten randomly chosen fields of vision. The area of the field of vision was about 60000 mcm2.
Results and discussion
First growth characteristics of the bacteria cultivated on the initial and modified PDMS samples during 24 hour period were studied. The results of the monitoring as the time dependencies of the microbe suspension optical density are presented on Fig. 2 and 3. Some differences in the growth dymamics of gram-positive and gram-negative strains were observed (Fig. 2 and 3 respectively).
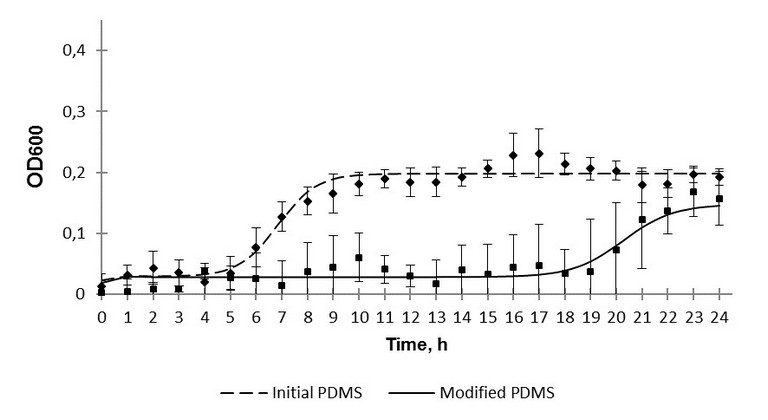
Fig. 2. The 24-hour growth of S. epidermidis over polydimethylsiloxane.
It should be noted that in presence of the modified samples ОD600 values are reliably lower those for the initial PDMS.
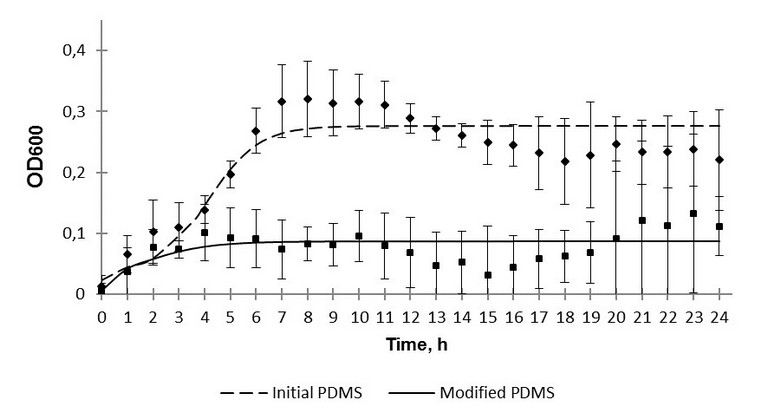
Fig. 3. The 24-hour growth of K. pneumoniae over polydimethylsiloxane.
Another fact worth mentioning is that in the case of the modified samples no reliable difference between optical density values after 24 hours of inoculation in dynamic and static regimes was observed. Optical density values in dynamic regime were 0,16 and 0,11, in static regime - 0,10 and 0,14 for S. epidermidis and K. рneumoniaе respectively. However, the corresponding values for the initial PDMS samples were significantly different: 0,19 and 0,22 in dynamic, 0,60 and 0,49 in static regime for S. epidermidis and K. рneumoniaе respectively. On the one hand, this might indicate antibacterial action of the modified surface. On the other hand, the static regime of bacteria cultivation can be assumed to be insufficient for a correct comparison of the effect of the silicon rubber surface with grafted fluorine-containing groups on different microbe cultures to be carried out. As silicon medical devices are often implanted for a long time, a prolonged monitoring of colonization of the initial and modified samples by the test-strains was of special interest. The sessile and plankton components of the bacterial cultures were monitored for 8 days. It was quite expected, that no reliable difference in optical density for the layers formed by different bacterial phyla adhered to the surface, or for the sessile component, was revealed. This phenomenon is evidently connected with an insufficient sensitivity of the method. According to the monitoring results, the influence of the initial PDMS on the plankton component can be neglected.
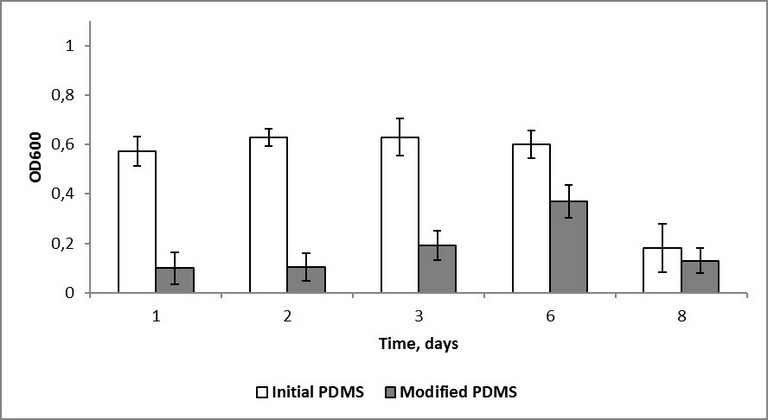
Fig. 4. Optical density of S. epidermidis plankton over polydimethylsiloxane.
In the case of the modified samples, the optical density values approached the ones registered for the initial samples, however, some delay was observed. In staphylococcus cultures no noticeable signs of biomass accumulation were observed. A decrease in staphylococcus optical density after 8 days is probably connected with a strongly adhered biofilm on the polymer surface.
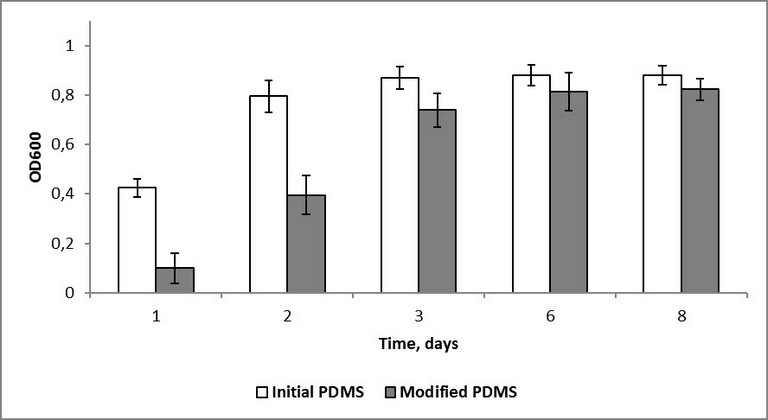
Fig. 5. Optical density of K. pneumoniae plankton over polydimethylsiloxane.
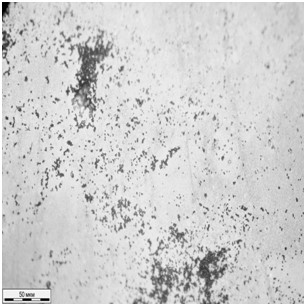
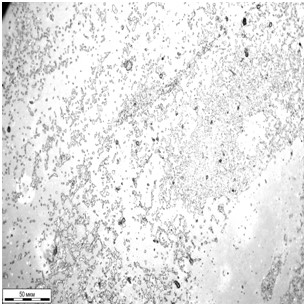
Traditionally, for a comparative estimation of biofilm formation, different versions of the technique described in [11] are used. It is assumed that only the microbe cells are dyed by gentian violet in contrast to the transparent polystyrene substrate. In this experiment, non-colonized areas remained colorless only in the case of initial samples. For the modified samples a uniform dyeing of the surface was observed due to chemisorption of the dyer molecules. So, photometric methods could not be used for estimation of biofilm formation, and instead microscopic methods were involved. The images of the initial and modified surfaces exposed to bacterial cultures for 8 days are shown in Fig. 6. Both for staphylococcus and klebsiella, the reduced intensity of biofilm formation on the modified surface is observed. The biofilm surface coverage was 23% and 14% for S. aureus and K. pneumonia respectively. There were no continuous fragments of bacteria held together by an extracellular matrix. In contrast to the initial surfaces, where the share of the fragments was 70% of the total biofilm area. Linear sizes of the continuous biofilm fragments varied from 20 to 200 mcm, and the biofilm surface coverage for initial PDMS surfaces was 40% and 56% in the case of S. epidermidis and K. pneumoniaе respectively. However, the images obtained using optical microscopy give the knowledge about a biofilm as a two-dimensional object, though biofilms are known to be three-dimensional ones.
a) |
b) |
c) |
d) |
Fig.6. The biofilms on the initial (a, c) and modified (b, d) PDMS samples after 8-day incubation of S. epidermidis (а, b) and K. pneumoniaе (c, d).
Nevertheless, according to the results obtained using the totality of the methods for the biofilm analysis, it can be stated that the suggested modification technique endows the silicon rubber antibacterial properties. This conclusion was supported by determination of the total protein content (TPC) in the adhered biomass. After 8 days of incubation TCP in the case of S. epidermidis was 0.19 and 0.73 mg/ml for the modified and initial surface respectively; and in the case of K. pneumoniaе - 0.17 and 0.36 mg/ml.
Conclusions
Thus, the method for modification of polydimethylsiloxane surface, aimed at antibacterial effect to the pathogens causing nosocomial infections, was suggested and tested. The perfluorinated hydrocarbon radicals were grafted on the surface macromolecules via a three-stage reaction. The study of bacterial growth by optical density monitoring for a 24-hour period showed an antibacterial effect to be pronounced in the case of S. epidermidis and moderate – in the case of K. рneumoniaе. The results of the prolonged contact of the bacterial cultures with the polymer samples were estimated using optical microscopy.The area of staphylococcus and klebsiella biofilms adhered to the modified PDMS samples was shown to be two and four times less than the one on the initial polymer surface, respectively. The total protein content determined in the biomass adhered to the sample surface furnishes additional strong support for antibacterial effect of PDMS surface with grafted fluorine-containing groups.
Acknowledgements
The work was supported by Russian Foundation for Basic Research (project №14-03-96013_ural_a)
References
- Chessal D., Ganaul G., Spiga L et al. // PLOS ONE. 2016. Vol. 11(1).
- Reśliński A., Mikucka A., Szmytkowski et al. // Pol J Microbiol. 2009. Vol 58(4). P.367–369.
- Reśliński A., Dąbrowiecki S., Głowacka K. // Med Biol Sci. 2014. Vol. 28(3). P. 35–42.
- Busscher H.J., Van der Mei H. C., Subbiahdoss G. et al. // Sci. Transl. Med. 2012. Vol. 4(153). P.1 –11.
- Pavlukhina S., Sukhishvili S. // Adv. Drug Deliv. Rev. 2011. Vol. 63(9). P. 822–836.
- Karpunina T.I., Yakusheva D.E., Kiselkov D.M. et al. Reducing Staphylococcus Epidermidis colonization of polydimethylsiloxane // Bulletin of Perm University. Biology. 2016. №2. P.160–165.
- Tomšič B., Simončič B., Orel B., Černe L. at al. //J Solgel Sci Technol. 2008.Vol. 47(1). P. 44 –57.
- Shao H., Meng W. D., Qing F. L. // J Fluor Chem. 2004. Vol. 125(5). P. 721–724.
- Nayak N., Ramprasad J., Dalimba U. // J Fluor Chem. 2016. Vol. 183. P. 59–68.
- Zhang Z., Wang J., Tu Q. et al. // Colloids Surf B Biointerfaces. 2011. Vol. 88(1). P. 85– 92.
- O’Toole G. A., Kaplan H., Kolter R. // Annu Rev Microbiol. 2000. Vol. 54. P. 49–79.
Recommended for publication by Prof. S. Igoumnov
Fluorine Notes, 2016, 109, 3-4