Received: August 2016
DOI 10.17677/fn20714807.2016.05.01
Fluorine Notes, 2016, 108, 1-2
PARTICULARITY PRIMARY PEROXIDE DERIVATIVE SYNTHESIS FROM REACTION FLUORINATED CARBONYL COMPOUNDS WITH HYDROGEN PEROXIDE
V. V. Chapurkina, V. P. Medvedeva, S. V. Chapurkinb
aVolgograd State Technical University, Lenina prosp., 28, Volgograd, 400131, Russia
E-mail:
chapurkin@vstu.ru
bZirax-Nefteservice LLC, Volgograd, Rokossovskogo st.,133, Volgograd, 400010, Russia
Abstract: At interaction of aliphatic, cyclic and aromatic fluorine-containing carbonyl compounds with hydrogen peroxide and hydroperoxides of tert-alkyles, 1- hydroxy-1- hydroperoxides and 1- hydroxy-1-tert-alkylperoxides were formed, hydro carbonic analogs of which mainly are not stable. Possibility of their extraction and further chemical transformations are being discussed.
Keywords: fluorine-containing, hydrogen peroxide, carbonyl compounds, hydroxyl hydroperoxides.
It is known that instability is common for peroxide compounds [1], and this limits the possibility of its’ practical application. The manufacturing industry avoided working with such compounds, that demand careful treatment, in every way. Frequently, there was ignored even the fact that only using one or another known peroxides, but which were not manufactured, it was possible to produce the materials with required properties. In fact, for a long time there have being and are now being used peroxides with obviously low properties, for example: benzoyl peroxide is widely used not only in manufacturing industry, but also in dentistry, but while it’s breakage toxic products are formed and an obtained polymer appears yellow in length of time, whereas there are known peroxides without these disadvantages [2].
Our article is dedicated to the peroxide synthesis on the base of poly- and perfluorocarbonyl compounds. Peroxide products of the reactions of carbonyl compounds with HOOH for many years are effectively used in larger organic scale preparation and in industry [3]. At the same time the synthesis of the 1- hydroxyl-1- hydroperoxides (НP) – the primary peroxides products of this reaction is a matter of permanent arguments because of its’ instability, when the property investigation of such primary HP is interesting for the investigation mechanisms of these reactions. Moreover, many oxidative processes of organic compounds flow through the mode of formation of similar primary hydroperoxides, that is why the primary products isolation is of particular interest.
When carbonyl compounds with hydrogen peroxide cooperation there is a mixture from different peroxides formed [1], and this is impossible to divide totally this mixture, and the most unstable of these peroxides is hydroxyl hydroperoxide.
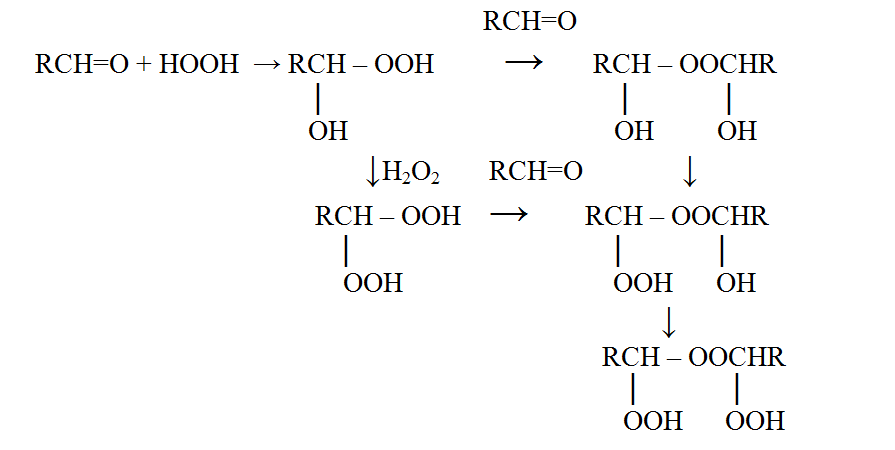
In the literature, there are constants of some peroxides, but the authors of the different articles give contradictory information and this is because no one managed to isolate it in an individual state [1-5]. The synthesis conditions, suggested by these authors, point that in the given conditions hydroxyl hydroperoxides should suffer further transformations, that is why in spite of the range of published works about its’ obtainment, no one managed to obtain it in a single form. This fact explains why there is not any work about the investigation of the properties of these hydroxyl hydroperoxides.
Among researches about the synthesis of peroxides based on different carbonyl compounds published recently, nowadays it is possible to depict the tendency that no one in general tries to isolate the hydroxyl hydroperoxides in the individual state [6].
The fluorine introduction into carbonyl compounds changes the situation dramatically.
We have investigated the reactions of a range of poly- and perfluorinated aldehydes with hydrogen peroxide and as a result fluorine-containing hydroxyl hydroperoxides were obtained [7].
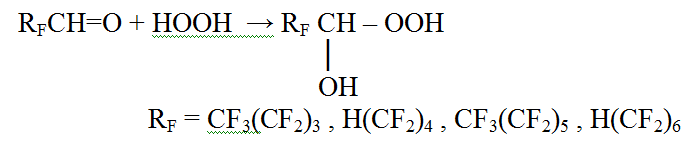
The fluorine atoms helped to stabilized this peroxide structure, more than, in case of perfluorinated radicals, hydroxyl hydroperoxides failed to further transformations, typical for the non-fluorinated compounds. The primary products of the reaction of perfluorocyclopentanone and perfluorocyclohexanone with HOOH were also obtained by us [8].
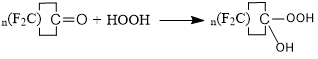
n=4, 5
We also managed to obtain hydroxyl hydroperoxides on the base of fluorine-containing aromatic aldehydes [9].

Ar = C6Н4, Х= Н , 3-F , 4- F , 2-CF3 , 3- CF3 , 4- CF3
Ar =C6F4 , Х= F
Research of such peroxides was of our interest because earlier such peroxides were not known.
First of all we checked the possibility of hydroxyl hydroperoxides in dioxyperoxides, that were earlier highly researched, transformation. Actually, the hydroxyl hydroperoxides connect the second aldehyde molecule with the dioxyperoxides formation, however, there was found that trifluoromethyl group in the aromatic nucleus complicates this reaction.
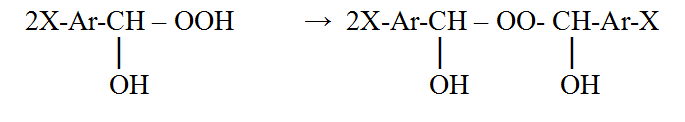
It seems that five fluorine atoms should show its high electron-accepting capacity and block this reaction, but such effect was not observed. The dioxyperoxide was obtained on the base of pentafluorobenzaldehyde, that shows the major difference in the transferring of the electronic influence of fluorine atoms in the nucleus and in the substitute.
Fluorine-containing hydroxyl hydroperoxides turned out to be stable compounds and we managed to analyze it, at that hydroxy and hydroxyperoxy groups are the subjects of acylation.
Considering that we managed to obtain the individual hydroxyl hydroperoxides, it was interesting to try to obtain mixed dioxyperoxide, because such peroxides in the literature were not described.
There was made a cooperation of the aromatic hydroxyl hydroperoxide on the base of о-trifluorobenzaldehyde with perfluoropentanal and perfluoroheptanal, however there was not observed the dihydroperoxides formation [10]. In this case there was obtained aliphatic hydroxyl hydroperoxide and о-trifluorobenzaldehyde was isolated.

We can assume that the more electron-seeking perfluoroaldehyde molecule separates the peroxy group from the aromatic hydroxyl hydroperoxide and the reaction flows through the intermediate cyclic state formation.
Unlike the known information, the formation of dihydroperoxides helps not carbonyl compound superfluity but hydrogen peroxide, what to our opinion can be explained by the hydrogen peroxide participation in the intermediate state formation that also corresponds with the quantum-chemical calculations data.
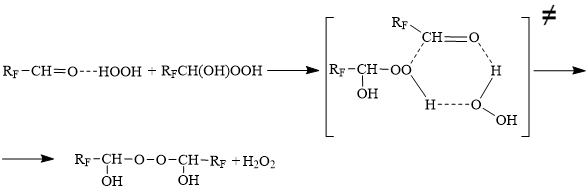
It has been established that fluorine-containing aromatic dihydroperoxides when resolving form carbonyl compounds [11].
The synthesis of primary products of the fluorinated carbonyl compounds with hydroperoxides tertiary alkyls reaction is also not without interest.
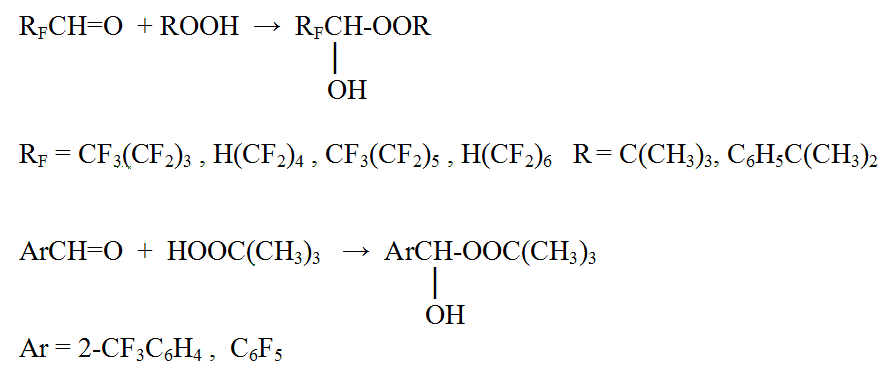
Perfluoroaliphatic aldehydes in this reaction form only stable hydroperoxides, when fluorine-containing aromatic aldehydes are able to form stable hydroperoxide only if there is a trifluoromethyl group in o-position or in case of complete replacement all fluorine atoms in the nucleus, in all other replaced benzaldehydes the reaction results in diperoxyacetals formation, in other words the more stable product in a consequence of second tert-butylperoxy group joining.
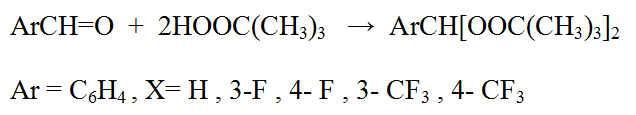
The fact that pentafluorophenyl group and trifluoromethyl group in the o-position prevents from joining the second tert-butylperoxy group can be explained by the high electron-seeking capability of these groups, that makes more difficult HO-group protonized finish in the 1- hydroperoxides and the further reaction behavior.
The main use of the fluoroperoxides is that they are the initiators of the polymerization and structuring processes, but considering the fact that the demands for these processes realization conditions may be different, we worked out the next ways of initialization for the given fluoroperoxides: thermal, with the usage of redox systems, UV and laser irradiance. The usage of all these methods lets to solve a range of major problems.
Fluorine-containing hydroxyl hydroperoxides are powerful initiators of polyester lacquer curing.
Polyfluoroalkyl peroxides let us to obtain transparent polymer materials with improved capability.
Application of synthesized fluoroperoxides for rubber structuring lets to raise vulcanizate’s thermal stability, chemical resistance and ozone resistance. It was stated that the application of testified elastomeric compositions of other known domestic and foreign initiators does not let to achieve such results [12]. In the tables 1 and 2 there is shown that the application of fluoroperoxides lets to raise the strength properties of a film in 3 times, but more important is that this lets to obtain transmission films that are able to bear inimical environments, for example gaseous fluorine effect or hydrofluoric acid effect.
Table 1
The films properties on the base of SKF – 260, structured by fluoroperoxides
PB and Di-tert-BPM
|
Peroxide |
The structure mode |
Conditions breaking strenghth, MPa |
Fineness ratio, % |
Remanent elongation, % |
|
|
Т 0С |
Time, min |
||||
|
Texturize peroxyde |
90 |
30 |
2,40 |
820 |
50 |
|
Texturize peroxyde |
90 |
30 |
2,62 |
960 |
40 |
|
PB |
120 |
30 |
0,82 |
760 |
100 |
|
Di-tert-BPM |
90 |
30 |
0,78 |
650 |
90 |
PB- Benzoyl peroxide
Di-tert-BPM – Di-tert-butylperoxymethan
Table 2. Gaseous fluorine effect (F2) on the films SKF-26 and hydrofluoric acid effect (HF) on the films SKF-32, structured by fluoroperoxides
|
Peroxide, 6 weight parts |
Swelling index, % |
The show of things, visual |
||
|
Film SKF-26, F2 |
Film SKF-32, 40 % HF |
Film SKF-26, F2 |
SKF-32, HF |
|
|
PB |
- |
11 |
Burnt |
swelled |
|
Peroximon |
1,8 |
- |
Got sunburnt |
- |
|
HTBPDPFH |
0,37 |
0,76 |
Unchanged |
Unchanged |
|
HTBPPFPM |
- |
0,64 |
- |
Unchanged |
Comments:
1.The gaseous fluorine effect 48 hour., 22 0С, pressure 1,12 ·105 N/m2 ; 2.The length of hydrofluoric acid effect 90 days., 22 0С.
HTBPDPFH - 1-hydroxy-1-tert-butylperoxy-1,7-dihydroperfluoroheptane
HTBPPFPM – 1- hydroxy -1- tert-butylperoxy -1-perfluorophenylmethan
The data received, shows that fluoroperoxides let to solve one more important problem concerning the surface protection from the environmental loads.
References
- Antonovskij V.L. //Organicheskie perekisnye iniciatory. M.:Khimiya. 1972. -447 s.
- Chapurkin V.V. Peroksidnye proizvodnye poli- i perftorirovannyh karbonil'nyh soedinenij. Sintez, svojstva i primenenie. Dis…. dok.khim.nauk. Volgograd. 1991.- 319 s.
- Ingram K. Identification of Antischistosomal Leads by Evaluating Bridged 1,2,4.5-Tetraoxanes, Alphaperoxides, and tricyclic Monoperoxides./ K.Ingram, I.A.Yaremenko, I.B.Krylov, L.Hofer, A.O.Ternt'ev, J.Keiser// I.Med.Chem. 2012. V.55. P.8700-8711.
- Milas N.. Some hydroperoxides / N. Milas, P. Panagiotakos // J.Am.Chem. Soc. 1946.V.68. N 3. P. 533-534.
- Terent'ev A.O. //Sintez geminal'nyh bisperoksidnyh soedinenij. V sb.3.:Sintezy organicheskih soedinenij. Pod red.akad.RAN M.P.Egorova, Maks-Press, g.Moskva. 2008. S.88-100;
- Terent'ev A.O. //Sintez i prevrashcheniya organicheskih peroksidov i reakcii s ispol'zovaniem peroksida vodoroda. Dis…. dok.him.nauk.Moskva. 2009.- 331 s.
- Chapurkin, S.V. Sintez ftorsoderzhashchih polikarbonil'nyh soedinenij i peroksidov na ih osnove / S.V. Chapurkin, V.V. Chapurkin, A.I. Rahimov // Vserossijskaya konferenciya po organicheskoj khimii, posvyashch. 75-letiyu so dnya osnovaniya In-ta org. khimii im. N.D.Zelinskogo: sb. tez. dokl. (Moskva, 25-30 okt. 2009 g.) / IOKh im. N.D.Zelinskogo RAN [i dr.]. - M., 2009. - C. 441.
- Rahimov A.I. O vzaimodejstvii perftorciklicheskih ketonov s peroksidom vodoroda/ A.I.Rahimov, E.M. Volynskaya, V.V. Chapurkin i dr // ZhOrKh. 1985. T.21, vyp.3. C. 656-657.
- Rahimov A.I. O sinteze oksigidroperekisej aromaticheskih al'degidov/ A.I.Rahimov, V.V. Chapurkin // ZhOrKh. 1978. T.14, vyp.1. S. 204.
- Chapurkin V.V. Sintez i osobennosti termicheskogo razlozheniya ftorsoderzhashchih aromaticheskih 1-gidroksi-1-gidroperoksidov i 1,1`-digidroperoksidov / V.V. Chapurkin, A.I. Rahimov, S.V. Chapurkin // Zhurnal obshchej khimii. - 2009. - T. 79, vyp. 2. - C. 254-257.
- Rahimov A.I., Chapurkin V.V. Novaya reakciya α-oksigidroperoksidov/ A.I.Rahimov, V.V. Chapurkin // ZhOrKh. 1981. T.17, vyp.7. S.1546-1547.
- Chapurkin V.V. Vulkanizatsiya ftorehlastomerov ftorperoksidami/ V.V. Chapurkin, V.P. Medvedev, S.V. CHapurkin // Zhurnal prikladnoj khimii. - 2015. - T. 88, vyp. 8. - C. 1161-1167.
Recommended for publication by Prof. S. Igumnov
Fluorine notes, Vol. 5(108) 2016
Fluorine Notes, 2016, 108, 1-2
