Received: June, 2016
DOI 10.17677/fn20714807.2016.04.02
Fluorine Notes, 2016, 107, 3-4
Synthesis and properties of the ε-aminocaproic acid polyfluorinated oligomers
A.V. Miroshnichenko., A.I. Rakhimov, N.A. Rakhimova
Volgograd State Technical University, Russia, 400131, Volgograd, Lenin
Prosp.,28
e-mail: organic@vstu.ru
Abstract: Oligomers of ε-aminocaproic acid with general formula H-[HN-(CH2)5-C(O)]m-OH (m = 60-80) were separated from the polycaproamide waste and were modified by the reactions with the polyfluorinated alcohols H(CF2CF2)nCH2OH and polyfluoroalkyl chlorosulfites H(CF2CF2)nCH2OS(O)Cl, n = 1, 2. The study of properties of obtained products (solubility, IR-spectra, elemental analysis) has shown that the main reaction direction of oligomers with polyfluorinated alcohols is N-polyfluoroalkylation and in case with polyfluoroalkyl chlorosulfites O – polyfluoroalkylation. There were obtained the polyfluoroalkylated oligomers with the fluorine content – 0.29-6.67% and with the molecular mass from 4600 to 9500.
Keywords: Oligomers of ε-aminocaproic acid, polyfluoroalkyl chlorosulfites, polyfluorinated alcohols, polyfluoroalkylation, polyfluoroalcoholysis.
It is known that oligoamides, same as polyamides have a range of goodness, such as high adhesion properties, hydrophobic nature, nontoxicity, oil-and-petrol resistance, durability of the polymer products and covers. Besides that, the reactive end groups existence in the oligomers structure makes possible their modification, that allows to give it new useful properties. As it was shown [1,2], an introduction of polyfluoroalkyl groups increases the hydrophobic properties of the oligomeric coatings and decreases its’ ability to inflammation. An addition of low-molecular polyfluoroalkylated oligomers in polycaproamide makes easier it's processing and increases the durability [3].
Earlier the polyfluoroalkyl ε-aminocaproic acid esters were achieved using the reaction of polyfluorinated alcohols with ε-caprolactam in conditions of catalysis by triethylamine and acids amides [2]. Using the calculative forecast method its biomedical activity summing up showed the high antiviral activity. The high antiviral activity of the oligomers of ε-aminocaproic acid was experimentally confirmed in the Research Institute of Influenza (Saint-Petersburg, professor Kiselev O.I.) [4].
In this regard, on account of the industrial accessibility of these oligomers, for us it seemed to be necessary to study the polyfluoroalcoholysis and polyfluoroalkylation of fractiones of ε-aminocaproic acid soluble in water.
There oligomer group of general formula H-[HN-(CH2)5-C(O)]m-OH was separated from the water using the waste products recrystallization. According to the end groups amount it was defined that m=60-80. Further the oligomer was modified by the polyfluorinated alcohols H(CF2CF2)nCH2OH and polyfluoroalkyl chlorosulfites H(CF2CF2)nCH2OS(O)Cl, n = 1, 2.
It is known that polyfluoroalkyl chlorosulfites are the high reactivity compounds, soft polyfluoroalkylation reagents for HO-alcohol group, phenols, carboxylic acids [5-8].
The polyfluoroalkyl chlorosulfites with oligomer reaction in the presence of triethylamine occurs in the soft conditions (the same for the carboxylic acids) and leads to the water soluble oligomers Ia and Ib formation. The six-membered polar intermediate state helps the nucleophilic substitution by reference to the α-methylene group polyfluoroalkyl chlorosulfites in the presence of carboxylic group:
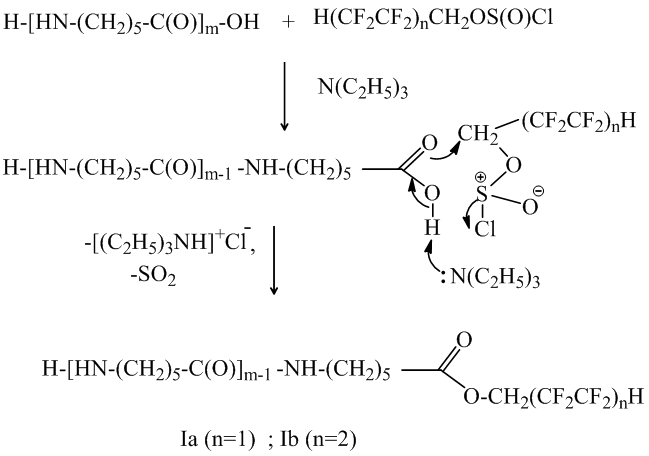
Further the reaction occurs with the water-insoluble N, O – di (polyfluoroalkyl) derivants of oligomers IIa and IIb formation:
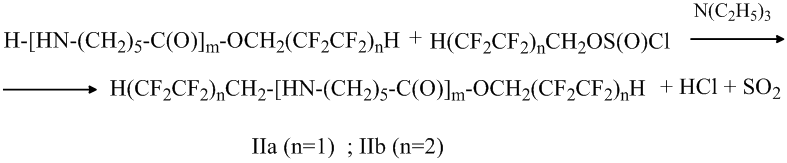
It seems that the oligocaproamide with the fluorinated alcohols reaction occurs in the following way:
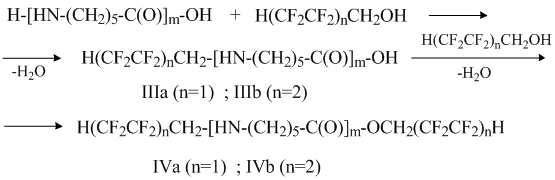
It is obvious that the mechanism of the N and O –polyfluoroalkylation of oligomer by the polyfluorinated alcohol is different from the mechanism of the reaction with polyfluoroalkyl chlorosulfite. The alcohol acidic nature and the law electron density at the oxygen atom of the HO-group makes the esterification of the oligomer’s carbonyl group more difficult and the cooperation may proceed with the amide bond destruction (that is why the oligomers’ III and IV molecular mass decreases), while the presence of the electrophilic center at the carbon atom CH2 – group promotes the attack of lone-pair electrons on nitrogen atom of terminal NH2 – group of oligomer’s molecule of this center. This confirms the fact that the main product of the polyfluorinated alcohol with oligomer reaction is the product of mono-N-polyfluoroalkylation – (IIIa, IIIb). The formation of the intermolecular hydrogen bond –F ∙∙∙ H- helps the transition structure stabilization:
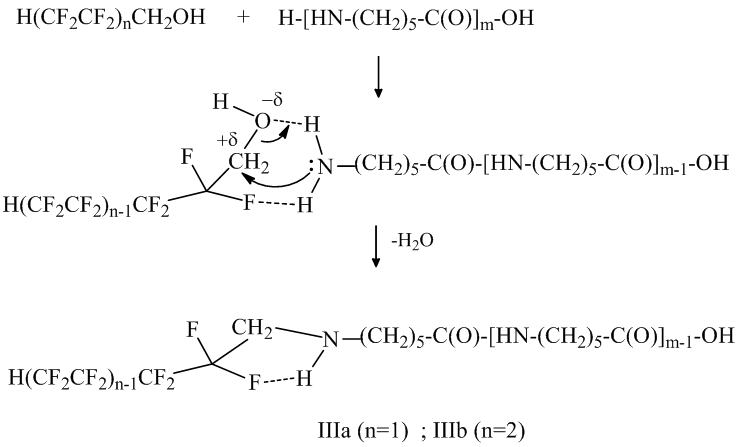
Formulas and the molecular mass of the achieved oligomers are shown in Table 1.
Table 1. The polyfluoroalkylated oligocaproamides.
# |
Formula |
Molecular weight |
Ia |
H-[HN-(CH2)5-C(O)]m-O-CH2CF2CF2H |
7380 |
Ib |
H-[HN-(CH2)5-C(O)]m-O-CH2(CF2CF2)2H |
9160 |
IIa |
HCF2CF2CH2-[HN-(CH2)5-C(O)]m-O-CH2CF2CF2H |
8350 |
IIb |
H(CF2CF2)2CH2-[HN-(CH2)5-C(O)]m-O- CH2(CF2CF2)2H |
9480 |
IIIa |
HCF2CF2CH2 H-[HN-(CH2)5-C(O)]m-OH |
7100 |
IIIb |
H(CF2CF2)2CH2-[HN-(CH2)5-C(O)]m-OH |
6520 |
IVa |
HCF2CF2CH2-[HN-(CH2)5-C(O)]m-O-CH2CF2CF2H |
6130 |
IVb |
H(CF2CF2)2CH2-[HN-(CH2)5-C(O)]m-O- CH2(CF2CF2)2H |
4560 |
The polyfluoroalkylated oligomers construction was researched using the IR-spectroscopy. The characteristic frequencies of absorption in the IR-spectra of the starting and polyfluoroalkylated oligomers are shown in the table 2.
In the large wave numbers range 3571-3272 cm -1 there was marked an intensive absorption band for the amides NH-groups with the frequency 3284-3272 cm -1, that according to its location and intensity changes in the small ranges. The absorption band 3116 cm -1 in the starting oligomer has the medium intensity. The analogical absorption is observed in the interval 3072-3066 cm -1 in the non-soluble in hot water oligomers IIa, IIb and IVa, IVb. The absorption band displacement is resulted from the NH-group association, which appears during the process of polyfluoroalkylation of the final oligomer’s NH2 – group with the fluorine atoms by the nearest CF2 – group:
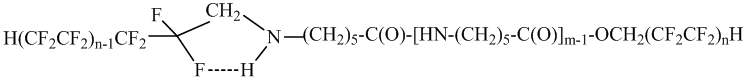
The 1176 cm -1 absorption band, which complies with asymmetrical deformation vibration of the C-F bond is the demonstration of the H(CF2CF2)2CH2 – group presence in the oligomer’s molecules (with exception of the fluorine molecule content). The absence of the CF2 – group association with H-N-group hydrogen atom in the oligomers Ia and Ib results in this absorption band displacement to the large wave numbers (1184 cm -1).
The presence of the ester group in the oligomers Ia, Ib and IIa, IIb is obvious because of its absorption in the stretch vibration sphere of the ester group with νC=O = 1748-1752 cm -1.
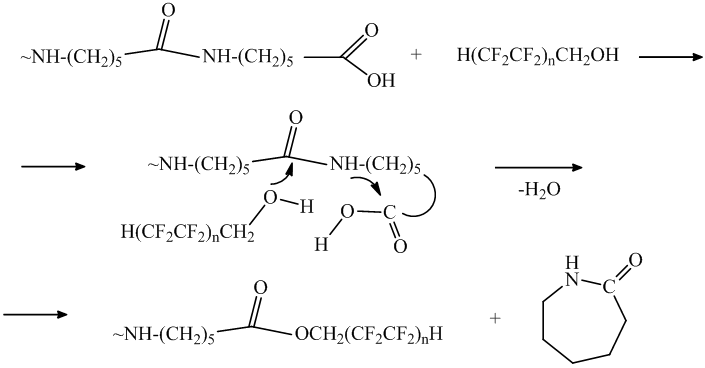
The difference of the fluorine content for the water soluble oligomers II and IV is attributable to their different molecular mass that is connected to the reaction polyfluorinated alcohol along the starting oligomer amide groups. The high fluorine content 5,48-6,67% in the oligomer IV, which was achieved by boiling in the polyfluorinated alcohol and therefore it’s lower molecular mass (4500-6100) is explained by alcoholysis of oligomer with amide bond destruction and ε-caprolactam or cyclic oligomers formation:
Polyfluoroalkylated oligomers I, III are white, crystal compounds, that are water soluble (fluorine content 1.03-1.66 and 1.07-2.33% accordingly). Increase of fluorine content in oligomers II and IV (1.82-6.67%) decrease its water solubility. Such oligomers is recommended for testing in the industrial rubber products for the purpose of decrease defacement while exploitation.
After the insoluble in the boiling water oligomer and the one dropping out while cooling till 20 °С were separated, from the water supernatant liquid after its concentration also drops out the oligomer that contents a small fluorine amount (0.29-1.0%). On the one hand the good water solubility can be explained by the presence of the cyclic oligomers, that are good water soluble and sublimating at high temperature, on the other hand, the law molecular oligomers with n ≤ 20-30 are good water soluble. The fluorine presence in this product lets to suggest that the product consists of polyfluoroalkylated through the amino group law molecular oligomer (the free carboxylic group helps its solubility) and of the cyclic non fluorinated oligomer.
Experimental.
The structure of the compounds obtained was confirmed by IR spectroscopy. The IR spectra were recorded on a Spekord – M82 instrument, in the suspension with the paraffinic oil.
1. The oligomeric waste of polycaproamide manufacture separation
The oligomers’ waste was powdered, treated by water at the temperature 20-25°С and then easily soluble ε-caprolactam and law molecular oligomers were separated. The cyclic oligomers were extracted using ethanol. The residue was boiled in the water and filtered at the temperature 80-90°С. The white, crystal oligocaproamide precipitated from the filtrate after cooling. Using the potentiometric titration method of the final H2N – and HOOC – groups it was defined that oligocaproamide H[HN(CH2)5C(O)]mOH has average m=60-80.
2. Oligocaproamide polyfluoroalkylation by the 1,1,5- trihydroperfluoropentyl chlorosulfite in the presence of triethylamine
In a reactor provided with a stirrer, thermometer, reflux condenser was put 2,5 g. (0.27·10-3 mole ) of oligomer, 0.6 ml (0.004 mole) of triethylamine and 15 ml of chloroform, the reaction mixture was cooled to -10°С and maintaining the temperature, the solution of 2.18 g (0.004 mole) 1,1,5- trihydroperfluoropentyl chlorosulfite in 5 ml of chloroform was fed. Than the temperature was slowly raised to 60-65 °С and exposed while stirring during 2 hours. The oligomeric product was filtered, washed with chloroform and recrystallized from the water. There was obtained: water insoluble product (IIb) 0.39 g, (15.6 %), MP 220-228°С, fluorine content 3.19 %; water soluble product (Ib) 0.93 g. (37.2 %), MP 225-227°С, fluorine content 1.66 % .
The reaction with 1,1,3- trihydroperfluoropropyl chlorosulfite was carried out similar, the results are shown in the Table 2.
3. The polyfluoroalkylation of oligocaproamide by the 1,1,5-trihydroperfluoropentan-1-ol
In a three-necked reactor provided with a stirrer, thermometer, reflux condenser was put 8 ml (0.05 mole) 1,1,5- trihydroperfluoropentan-1-ol and 4.95 g. (0.54·10-3 mole) of oligocaproamide. The reaction mixture was heated to boiling and allowed during 12 hours. After the reaction mixture was cooled, it was diluted with 20 ml of acetone. The precipitated white substance was filtered, and using the boiling water soluble part was extracted. The water insoluble oligomer IVb in amount of 0.59 g. (11.9 %) with MP 215-220 °С and the fluorine content - 6.67 % was obtained. After cooling water solution 2.26 g. (45.7 %) of oligomer IIIb with MP 232-240 °С and fluorine content equal to 2.33 % was obtained. The reaction with 1,1,3- trihydroperfluoropropan-1-ol was carried out similar, the results are shown in the Table 2.
Table 2. Physico-chemical properties of the initial and polydluoroalkylated oligomers.
|
No |
Olygomer |
Yield, % |
MP, ºC |
Content of Element, % |
Solubility in boiling water |
The characteristic frequencies in the IR spectra, cm -1 |
|||||
|
NH amido group |
NH (NH2) amine |
C(O)ORF ester group* |
COOH |
CF2 |
|||||||
|
N |
F |
||||||||||
|
1 |
initial |
- |
220-222 |
12.2 |
- |
soluble |
3284 |
(3116) |
- |
1716 |
- |
|
2 |
Ia |
24.8 |
235-240 |
12.1 |
1.03 |
soluble |
3286 |
3084 |
1748 |
- |
1183 |
|
3 |
Ib |
37.2 |
227-235 |
12.0 |
1.66 |
||||||
|
4 |
IIa |
10.0 |
230-235 |
9.9 |
1.82 |
insoluble |
3271 |
3067 |
1746 |
- |
1177 |
|
5 |
IIb |
15.6 |
220-228 |
9.8 |
3.19 |
||||||
|
6 |
IIIa |
45.6 |
230-235 |
11.8 |
1.07 |
soluble |
3280 |
3076 |
- |
1716 |
1176 |
|
7 |
IIIb |
45.7 |
232-240 |
10.9 |
2.33 |
||||||
|
8 |
IVa |
13.2 |
235-245 |
8.9 |
2.48 |
insoluble |
3280 |
3072 |
1752 |
- |
1176 |
|
9 |
IVb |
11.9 |
215-220 |
9.9 |
6.67 |
||||||
References
- Storozhakova N.A. Modification of poly-e-caproamide by polyfluorinated alcohols-telomers. Autor’s abstract of Dissertation. Volgorad.1998.
- Efanova E.Ju. Catalitic reactions of caprolactam in synthethis of oligomers. Autor’s abstract of Dissertation. Volgorad.2007.
- Kosenkova S.A. Conformity investigation for catalytic reactions of e-caprolactam with alcohols. Autor’s abstract of Dissertation. Volgorad.2007.
- Kiselev O.I., Deeva E.G. Antivirus preparations. Review. Institute Grippe. Sanct-Peterburg, Kazan. 2001.32 p.
- Rakhimov A.I. Synthesis of polyfluoroalcylsulfites and new reaction of polyfluoroalcylation with their participation. J. Gen.Chem.2010, vol.80, p.1315-1334.
- Rakhimov A.I., Vershinin O.A., Miroshnichenko A.V. Influence of polyfluoroalcyl groups in copolymer of acrilamide with acrilate natrium on properties of little concentrated water solutions. Fluorine Notes.2011, 4.
- Rakhimov A.I., Miroshnichenko A.V. Specific synthesis of phenylethers with using polyfluorochlorcylsulfites. Fluorine Notes.2011, 6.
- Rakhimov A.I., Nikishin G.I., Miroshnichenko A.V., Phiong Tehao Do Iiong. Influence of substationes in allilic alcohole on reaction with polyfluorochloro sulfites. Fluorine Notes.2011, 5.
Recommended for publication by Prof. A. I. Rakhimov
Fluorine Notes, 2016, 107, 3-4
