Received: June, 2016
DOI 10.17677/fn20714807.2016.03.04
Fluorine Notes, 2016, 106, 5-6
Thermal degradation of silver 2-hydroperfluoro-3-methylcrotonoate
M.G. Medvedeva, V.F. Cherstkova, N.D. Kagramanova, A.A. Tyutyunovab, S.M. Igumnovab, S.R. Sterlina
aA.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, V-334, GSP-1, 119991 Moscow, Russia
bNPO PiM-INVEST LLC, ul. Vavilova 28, 119991 Moscow, Russia
e-mail: sterlins@yandex.ru
Abstract: It is shown that 1,1,1,6,6,6-hexafluoro-2,5-bis(trifluoromethyl)-2,4-hexadiene – the main product of silver 2-hydroperfluoro-3-methylcrotonoate thermolysis –undergoes prototropic rearrangement and dehydrofluorination under the reaction conditions to give a mixture of polyunsaturated compounds.
Keywords:silver 2-hydroperfluoro-3-methylcrotonoate; 1,1,1,6,6,6-hexafluoro-2,5-bis(trifluoromethyl)-2,4-hexadiene; tetrakis(trifluoromethyl)butatriene; 1,2-bis(perfluoropent-2-yl)acetylene.
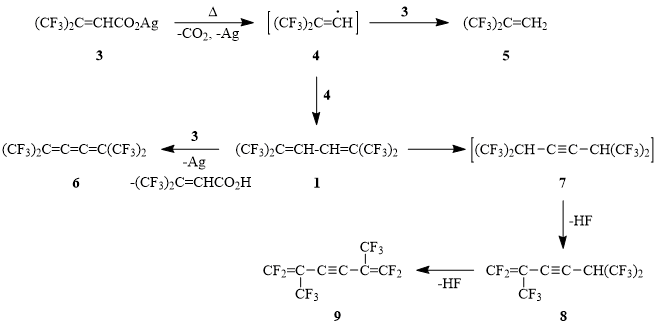
Earlier in the course of study devoted to the synthesis of gem-bis(trifluoromethyl) substituted olefines and dienes it was shown that 4,4,4-trifluoro-3-(trifluoromethyl)-2-butenal reacts with hexafluoropropylidenetriphenylphosphoran generated in situ to give 1,1,1,6,6,6-hexafluoro-2,5-bis(trifluoromethyl)-2,4-hexadiene (1) [1].
It is known that degradation of silver salts of perfluoro-α,β -unsaturated carboxylic acids of general formula RFCF=CFCO2Ag (RF = i-C3F7; tert-C4F9) (2) at 210-220°С leads to the formation of the corresponding α-dienes in 80-85% yields [2]. It could be assumed that thermolysis of silver 2-hydroperfluoro-3-methylcrotonoate (3) would appear to be a convenient method of preparing diene 1.
We have found that thermal degradation of salt 3 at 170-240°С/10-12 Torr leads to the formation of product mixture that contains, along with the expected diene 1, compounds 5, 6, 8, 9 which structures were suggested based on GC-MS-data. The comparison of molecular ions of compounds 1, 6, 8, 9 leads to the evident conclusion that the compounds 6-9 are the diene 1 derivatives, formed either as a result of its oxidation (butatriene 6) or sequential mono- or didehydrofluorination (propenyl acetylene 8 and bis(propenyl) acetylene 9).
Hence it appears that diene 1 is in fact the main product of salt 3 thermal degradation, and complex mixture of the reaction products is determined by oxidation of two C-H-bonds in 2,3-positions of butadiene fragment of the molecule under the action of Ag+-ions as well as prototropic migration of hydrogen atoms that leads to the intermediate formation of bis(hexafluoroisopropyl) acetylene 7 – precursor of acetylenes 8-9.
Most probably the driving power of prototropic allylic rearrangement of α-diene 1 is a high polarization of double bonds under the influence of four CF3-groups in 1,4-positions of butadiene fragment. The quantum-chemical calculation shows that enthalpy of acetylene 7 formation as a consequence of diene 1 prototropic isomerisation is 14 kJ/mol. Taking into consideration that decarboxylation of crotonoate 3 begins at 170°С the formation of acetylene 7 as the intermediate appears to be highly probable.
Scheme 1
Experimental
Mass spectra are recorded using a Finnigan Polaris Q mass spectrometer (Trace GC ultra).
Quantum-chemical calculations were performed by TPSSh/6-311++G(d, p) method assisted by program complex GAMESS-US at supercomputer BlueGene/P of CM&C faculty (Moscow University) [4, 5]. According to calculation data the enthalpy of diene 1 isomerization into acetylene 7 is 14 kJ/mol. The gauche conformation of appeared to be energetically favorable.
Thermolysis of salt 3.
Sodium carbonate (3.76 г, 35.5 mmol) was added to solution of 16 g (71 mmol) 4,4,4-trifluoro-3-(trifluoromethyl)crotonic acid monohydrate in 100 ml of water, when the evolution of CO2 ceased the solution of 12.05 g (71 mmol) AgNO3 in 20 ml of water was added under stirring, the precipitate was extracted with ether, the extract was evaporated, the residue (salt 3) was dried over P2O5 at 100°С/1-2 Torr. The salt 3 obtained (19.2 g, 61 mmol) was heated at 170-240°С/10-12 Torr, the volatile products were collected into receiver (-78°С). According to CG-MS-data the distillate (8.2 g) for ~90% consists of compounds 1 : 5 : 6 : 8 : 9 in approximate ratio 10 : 5 : 5 : 1 : 3. The attempt to fractionate the mixture obtained led to intensive tarring; no analytically pure compounds were isolated.
Mass-spectrum of 1 (m/z, reference): 326[M]+; 307[M-F]+; 287[M-H-2F]+; 257[M-CF3]+; 237[C7HF8]+; 219[C7H2F7]+; 207[C6H2F7]+; 191[C8F5]+(100%); 181[C7H2F5]+; 163[C4HF6]+; 113[C3HF4]+; 75[C3HF2]+; 69[CF3]+ (cf. [1, 8]).
Mass-spectrum of 5 (m/z, reference): 164[M]+; 163[M-H]+; 145[M-F]+(100%); 113[C3HF4]+; 95[M-CF3]+; 75[C3HF2]+; 69[CF3]+.
Mass-spectrum of 6 (m/z, reference): 324[M]+; 305[M-F]+; 286[M-2F]+; 267[C8F9]+; 255[M-CF3]+(100%); 236[C7F8]+; 217[C7F7]+; 205[C6F7]+; 186[C6F6]+; 167[C6F5]+; 148[C6F4]+; 117[C5F3]+; 69[CF3]+ (cf. [6, 7]).
Mass-spectrum of 8 (m/z, reference): 306[M]+; 287[M-F]+; 267[C8F9]+; 237[M-CF3]+; 218[C7HF7]+; 199[C7HF6]+; 187[C6HF6]+; 168[C6HF5]+(100%); 148[C6F4]+; 137[C5HF4]+; 117[C5F3]+; 99[C5HF2]+; 93[C3F3]+; 75[M-CF3]+; 69[CF3]+.
Mass-spectrum of 9 (m/z, reference): 286[M]+(100%); 267[M-F]+; 236[C7F8]+; 217[C7F7]+; 186[C6F6]+; 167[C6F5]+; 148[C6F4]+; 129[C6F3]+; 117[C5F3]+; 98[C5F2]+; 79[C5F]+; 69[CF3]+; 43[C2F]+.
References
- A. Haas, M. Lieb, J. Zwingenberger, Liebigs Ann., 1995, 2027-2035.
- V.F. Cherstkov, M.V. Galakhov, E.I. Mysov, S.R. Sterlin, L.S. German, Bull. Acad. Sci. USSR, Div. chem., sci., 1989, 38, 1219-1223.
- I.L. Knunyants, Ch’en Ch’ing-yun, N.P. Gambaryan, Zh. VKhO im. Mendeleeva, 1960, 5, 112-113.
- J. Tao, J.P. Perdew, V.N. Staroverov, G.E. Scuseria, Phys. Rev. Lett., 2003, 91, 146401.
- M.S. Gordon, M.W. Schmidt in Theory Appl. Comput. Chem. (Eds.: G.E. Scuseria, C.E. Dykstra, G. Frenking, K.S. Kim), Elsevier, Amsterdam, 2005, 1167–1189.
- P.A. Morken, D.J. Burton, Synthesis, 1994, 969-972.
- R.N. Warrener, E.E. Nunn, M.N. Paddon-Row, Tetrahedron Lett., 1976, 2639-2642.
- T.P. Forshaw, A.E. Tipping, J. Chem. Soc., C, 1971, 2404-2408.
Recommended for publication by Prof. S. Sterlin
Fluorine Notes, 2016, 106, 5-6
