Received: November, 2015
DOI 10.17677/fn20714807.2016.03.03
Fluorine Notes, 2016, 106, 3-4
Quantum-chemical assay of phosgenation of polyfluorinated alcohols catalyzed by N,N –dimethylformamide
A.I. Rakhimov1,2, A.O. Litinskiy 1, S.S. Jukov 1, L.A. Butkovskaya1, N.A.Rakhimova1, V.V. Moskwa 3
1Volgograd State Technical University, 400131, Russia, Volgograd, Lenin prospect, 28
e-mail: organic@vstu.ru
2Institute of Chemical problems of Ecology ANS RF, 400066, Volgograd PO box 127,
e-mail: rakhimov@sprint-v.com.ru
3Dmitry Mendeleev University of Chemical Technology of Russia, 125047, Russia, Moscow,Miusskaya sq., 9. e-mail: organic@distant.ru
Abstract: Using the method of density functional theory (DFT) tool by applying an exchange – correlation density functional B3LYP and basis set LANL2DZ according to the programme MOLPRO, there was made a quantum-chemical assay of phosgenation of polyfluorinated alcohols catalyzed by N,N – dimethylformamide. Form factors of each compound and equilibrium geometries associate parent molecule were searched by minimizing total energy. As one can see catalyst role concludes in phosgene and alcohol molecules polarization, that helps the process flowing.
Keywords:polyfluorinated alcohol, phosgene, polyfluoroalkyl chloroformiate, N, N-dimethylformamide, density functional theory (DFT), the programme MOLPRO.
The development of methods of microdoping of polyfluorinated fragments into macromolecular schemes is an important task as it allows significant improving of physical and chemical properties of polymer composition materials [1-11]. One of introduction methods of polyfluoroalkyl groups is application of polyfluoroalkylchloroformiates – reagents for obtaining polyfluoroalkyl-peroxydicarbonates used as initiators and modifiers for polymer materials for these purposes, they differ by their high resistance to impact of strong oxidizers and aggressive media, non-combustibility, thermal stability and necessary durability characteristics [12, 13].
In the synthesis of polyfluoroalkyl-peroxydicarbonates high toxic phosgene is used, that is why it is important using quantum-chemical research tool to choose an effective catalyst of phosgenation. Out of all polyfluorinated alcohols being researched, the most effective were catalyzed by N,N-dimethylformamide (in comparison with trimethylamine and pyridine). The reaction is going according to the following scheme:
H(CF2)nCH2OH + COCI2 → H(CF2 )nCH2ОС(О)CI + НСI
The influence of the precursor phosgene and the difluoromethylene groups in the alcohol molecule on this reaction was finding out by comparing of electronic characteristics of the precursors (phosgene, propanol) H(CF2)nCH2OH and tetrafluoropropanol H(CF2)nCH2OH (and it’s transmutation products). The electronic structure of the phosgene molecule is shown in the picture 1:
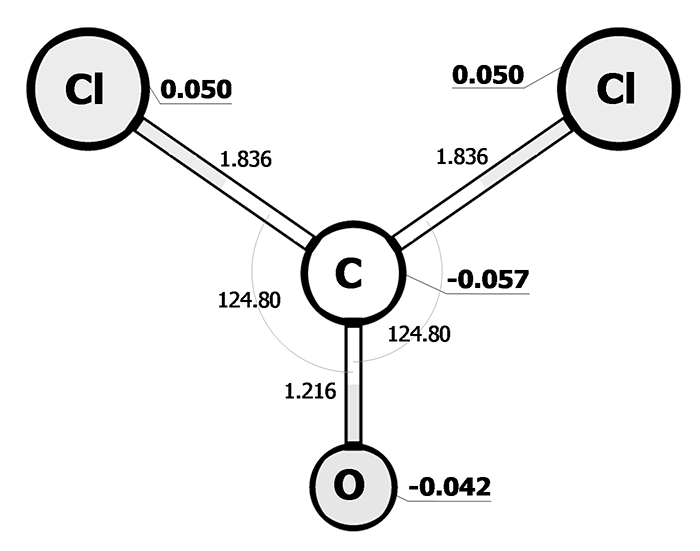
Pic. 1: The electronic structure of the phosgene molecule.
As it is shown in the picture 1, the introduction of electron seeking chlorine atom displaces the electron density, that is equal to – 0, 057, to the carbon atom. Hydroxyl group is the most polar in the propanol molecule (pic. 2) (-0.461 on an oxygen, and 0, 340 on a hydrogen).
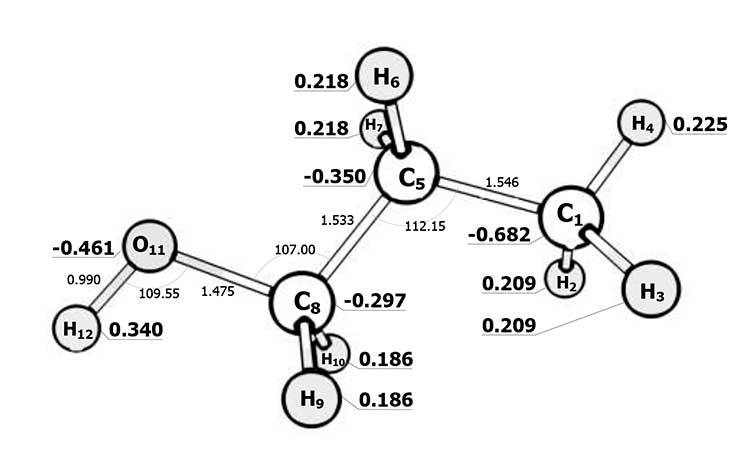
Pic. 2. The electronic structure of the 1-propanol.
High polarity of N, N-dimeythylformamide and formaldehyde groups is the unique feature of its electronic structure (pic. 3). As opposed to propanol, 2.2.3.3-tetrafluoropropanol (pic. 4) is more polar (charge – 0,462 on an oxygen and 0,366 on a hydrogen of hydroxyl group).
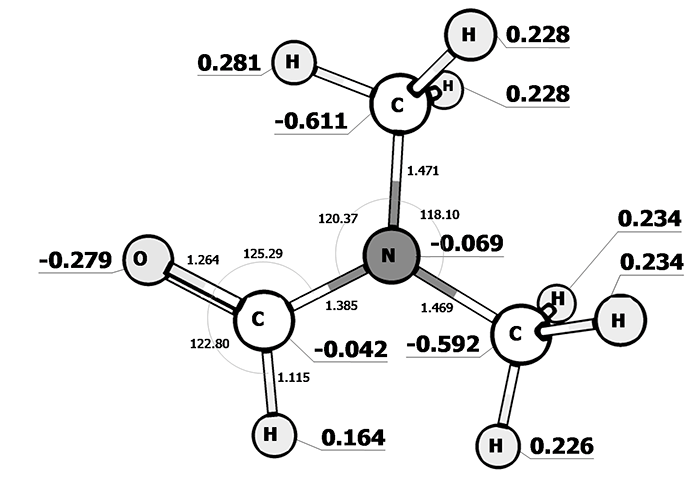
Pic. 3: The electronic structure of N, N-dimeythylformamide.
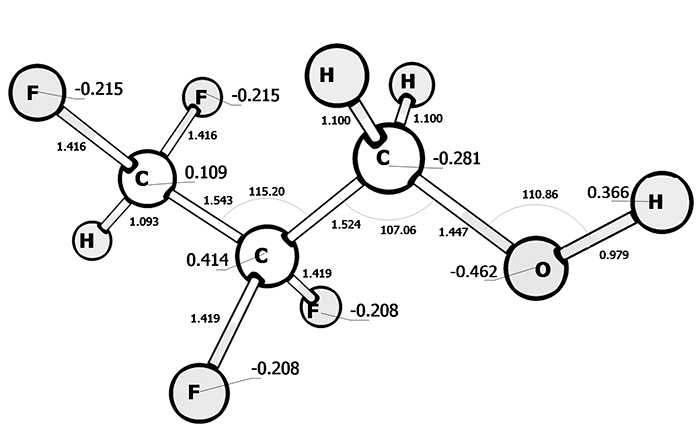
Pic. 4: The electronic structure of 2.2.3.3 tetrafluoropropanol.
In the pic. 5 you can see the charge distribution on the molecule’s way to the pre-reaction complex phosgene – 2.2.3.3. – tetrafluoropropanol and N, N-dimeythylformamide.
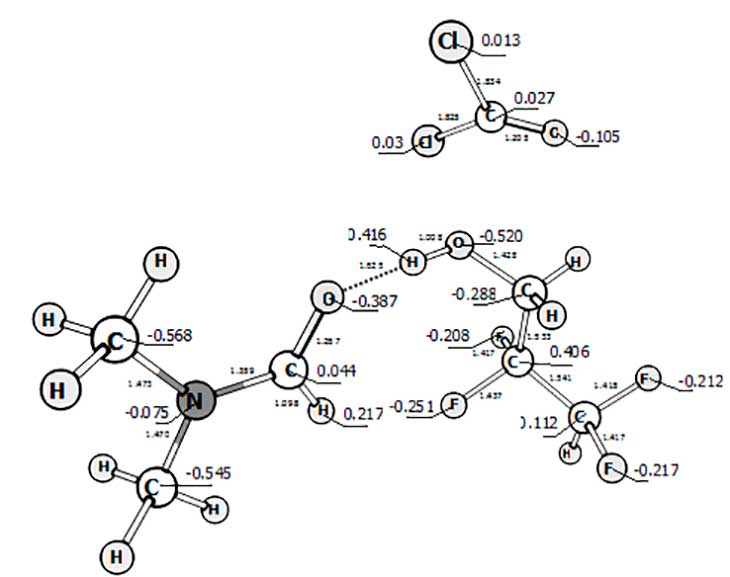
Pic. 5: The charge distribution in the pre-reaction complex 2.2.3.3. – tetrafluoropropanol and phosgene, catalyzed by N, N-dimeythylformamide.
The catalysts role (N,N-dimethylformamide) is to take part in polarization of phosgene and alcohol molecules, that helps reaction behavior. Let us suppose the building up an intermediate state, that includes molecules of alcohol, phosgene, catalyst and as a result subsequent formation of carbonochloridate.
One type of such contacting is shown in the picture given below, that includes six-membered intermediate state:
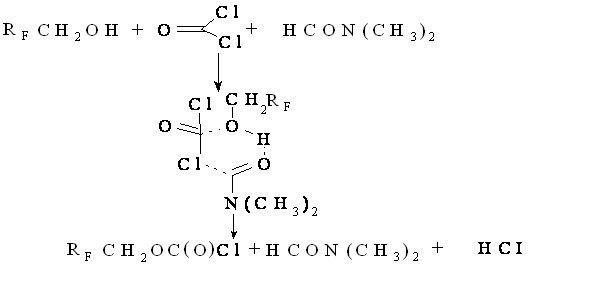
It should be noted that the energy of the forming associate is less than the energy of the parent molecule on 1, 79 eV and is equal to 173,11 KJ/mol.
Conclusion
Using the method of density functional theory (DFT) tool by applying an exchange – correlation density functional B3LYP and basis set LANL2DZ according to the programme MOLPRO, there was made a quantum-chemical assay of phosgenation of polyfluorinated alcohols catalyzed by N,N – dimethylformamide. The influence of introduction the fluorine atom into the molecule of alcohol and its chemical reactivity were discussed.
References
- Rakhimov A.I. Methods doping of polyfluoro- groups into macromolecular systems. New perspective materials and technologies. Collection of scientific works 5- International conference. Volgograd. 2010, p.79-80.
- Rakhimov A.I. Chemistry and Technology of fluoroorganic compounds. M. Chemistry.1986. 271p.
- Rakhimov A.I., Krukova E.G., Bogath E.V. Sergeev S.A., Kostirja V.I. Pat.2021286. R.F. MPK S1F224.114/08.1994.
- Rakhimov A.I., Bogdanova O.S.,Butkovskaj L.A. Obtaining of suspension polyvinylchloride with help polyfluoroperoxydicarbonates. Chemistry and polymeric materials. Collection of scientific works of Volgograd State Technical University. Volgograd. 1999.p. 78-88.
- Rakhimov A.I. Chemistry and Technology of organic Peroxides. M. Chemistry.1979. 389 p.
- Rakhimov Alexander. Initiators for Manufacture of PVC. New York. Nova Science Publishers. 2008. 181 p.
- Rakhimov A.I., Butkovskaj L.A., Baklanov A.V. Synthesis of polyfluoro – tert. – butylperoxyalkylcarbonates. Chemistry and Technology element organic monomeric and polymeric materials. Volgograd .2008, v.39, #1, p.85.
- Rakhimov A.I., Butkovskaj L.A. Particularities of thermolysis for di(polyfluoroalkyl) peroxydicarbonates and theirs application. Fluorine Notes, Vol. 3(82), 2012.
- Rubanova R.A., Androsuk E.R., Rakhimov A.I. Investigation of activity for fluorobenzoyl peroxides in process of structured elastomers. Volgograd conference.1980, 300 p.
- Rakhimov A.I., Butkovskaj L.A. The influence of dimethylenoxide group in polyfluoroalkylperoxydicarbonates on destraction Process.. Fluorine Notes , Vol. 2(87), 2013.
- Rakhimov A.I., Butkovskaj L.A. Synthesis and properties of di(polyfluoroalkyl peroxy)dicarbonates. J.General Chemistry 2011,v.81,#5, p.889.
- Galyl- Ogli F.A.,Novikov A.S., Nudelman Z.N. Fluorocaoutchoucs and rubbers on them base. M.Mir,1975, 240-260 p.
- Pasiorerek K. Structuretion of caotchoucs. In Fluoropolymers. M. Mir.1975.
- Orlov S.I. , Chimistkjn M.S. Kinetica and mechanism fosgenation of aliphatic alcohols. J.General Chemistry 1983,v.9,#11, p.2266-2271.
Recommended for publication by Prof. A. Rahimov
Fluorine Notes, 2016, 106, 3-4
