Received: April, 2016
DOI 10.17677/fn20714807.2016.03.02
Fluorine Notes, 2016, 106, 1-2
Synthesis of bromopolyfluoroarenes from polyfluoroarenethiols with PBr5 and Br2
P.V. Nikul'shin, A.M. Maksimov, V.E. Platonov*
N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry of the Siberian Branch of Russian Academy
of Sciences,
pr. Akademika Lavrent,eva 9, Novosibirsk, 630090 Russia
e-mail:
platonov@nioch.nsc.ru
Abstract: Using the reactions of the polyfluoroarenethiols with PBr5 and Br2 at 150-240°C in ampules and with Br2 in a flow system at 350-650°C, there were made the substitute reactions of thiol group by bromine atom and there was synthesized a variety of bromopolyfluoroarenes, among which there are also bromopolyfluoroarenes that at the same time contain chlorine and bromine atoms in ortho – and para- positions.
Keywords: Bromine, bromopolyfluoroarenes,polyfluoroarenethiols, thiol group
The thiol function is easily and selectively introduced into polyfluoroarenes by reaction of nucleophilic substitution [1]. Earlier we developed a method of introducing bromine and chlorine atoms into polyfluoroarenes by thermal replacement of a thiol group into polyfluoroarenethiols for chlorine and bromine atoms [2,3]. This process occurs at high temperature (400-500°C) in a flow system and leads to a variety of chloro – and bromopolyfluoroaromatic compounds with the high yield of compounds [2,3]. When using PCl5 as the source for chlorine, the substitute reaction of thiol group was made at lower temperature (200-220°C, reactions in ampules) [4]. In that context it is of interest to make such transformations of the polyfluoroarenethiols with PBr5 and Br2.
We have shown that the thiol group in polyfluoroarenethiols when heated at ~220°C in ampules with PBr5 or Br2 converts to bromine atom. In that way from the pentafluorobenzenethiol 1 and PBr5 (in the molar ratio of 1:2) at ~220°C during 8 hours bromopentafluorobenzene (2) with high yield was prepared (scheme1). In the pentafluorobenzenethiol (1) reaction, there can be used Br2 instead of PBr5. Thiol (1) when heated with Br2 (in the molar ratio of ~1:4.5) at ~220°C during 8 hours in this case arene 2 and decafluorodiphenyl disulfide (3) are formed (in the molar ratio of ~84 : 16 according to 19F NMR data). By increasing the time of the reaction till 16 hours the compound 2 was obtained with high yield (scheme 1).
Scheme1
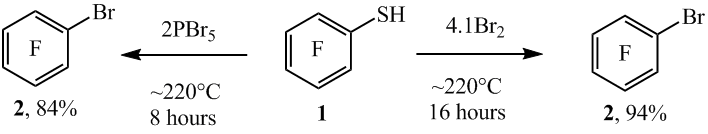
Reducing the
amount of Br2 to 2 moles, and reaction time to 5 hours resulted in the formation of arene 2 and disulfide 3 with the yields 44% and 48% respectively (according to GLC, scheme
2). Under similar conditions reactions of thiol 1 with Br5 (in the molar ratio of
~1:2) or PBr3 + Br2 (in the molar ratio of ~1:2:2) the compound
2 is formed and this compound contains small amounts of disulfide 3 (19F NMR and GLC). In the reactions of the thiol 1 with PBr3 (in the molar ratio of ~1:2) only the compound 3 is formed.
Scheme2
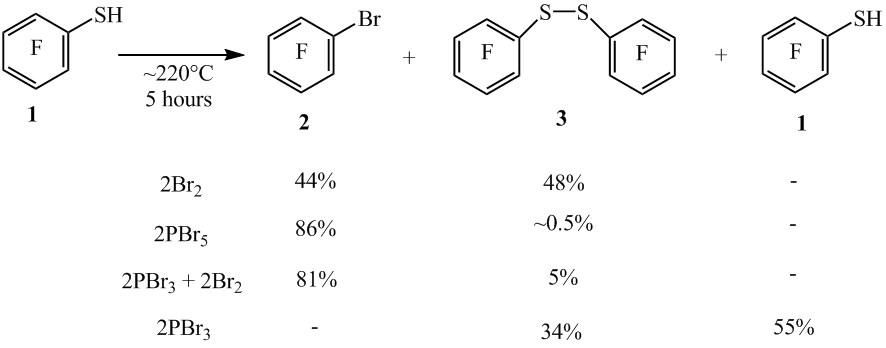
In case of increasing the reaction of 1 time with Br2 (in the molar ratio of ~1:2) to 8 and 16 hours, the reaction didn’t proceed fully and the reaction mixtures contained the disulfide 3 (scheme 3). By increasing the time of the reaction to 22 hours the arene 2 was obtained with the yield of 89%.
Scheme3
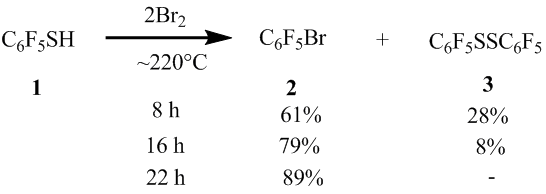
In the reaction of thiol 1 with Br2 in the molar ratio of ~1:3 at 220°C reaction during 16 hours, the yield of the compound 2 was 91%. As stated above, in the similar conditions, from thiol 1 and Br2 (in the molar ratio of ~1:4.1) there was obtained the compound 2 with the yield of 94%. Although there isn’t much difference between the yields of the target products in these reactions, in order to get the maximum yields of the final products, for further transformations of polyfluoroarenethiols with bromine, we chose their molar ratio of ~1:4-4.3.
In case of increasing temperature of the reaction of the compound 1 with Br2 (in the molar ratio of ~1:4.1) to 250°C (16 hours) an arene 2 is obtained with small impurity of polybromopolyfluorobenzenes, according to 19F NMR, GC-MS and GLC (table 1, entry 7). Earlier in co-pyrolysis of C6F6 with Br2 at ~770°C arene 2 and polybromopolyfluorobenzenes [5] were formed. In this regard, the temperature ~220°C is acceptable for bromination of the compound 1 and its derivatives.
When heated disulfide 3 with bromine at ~220°C also arene 2 with high yield is obtained (scheme 4).
Scheme4
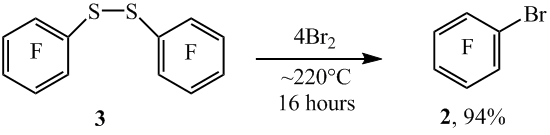
In case of thiol’s 1 para-substituted, the thiol group is also smoothly substituted by bromine. In such way 1-bromo-2,3,5,6- tetrafluorobenzene (5) is obtained by heating 2,3,5,6-tetrafluorobenzenthiol (4) with bromine at ~220°C. In this case, the C-H bond remains unreacted. Much the same there was 1,4-dibromotetrafluorobenzene (7) obtained from 4-bromotetrafluorobenzenethiol (6) (scheme5).
Scheme5
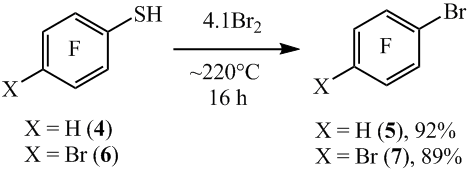
4-Chloro-2,3,5,6-tetrafluorobenzenethiol (8) when heated with bromine at ~220°C, than apart from 1-bromo-4-chloro-2,3,5,6-tetrafluorobenzene (9) arene 7 (scheme 6) is formed as a co-product in virtue of the part substitution of chlorine atom by the bromine. A decrease of temperature in the reaction to ~180°C allowed to decrease the content of the compound 7 (scheme6).
Scheme 6
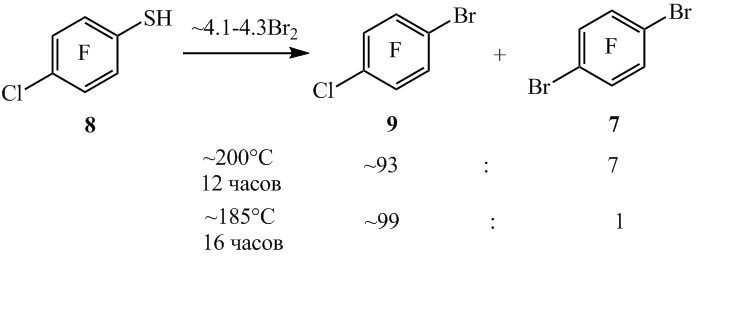
mole ratio of reaction products in reacting masses calculated from GLC data
The were made co-pyrolysis reactions of thiol 8 with Br2 in a flow system at different temperatures. At 350°C from thiol 8 and Br2 there was got arene 9 with the smaller yield and with an impurity of the compound 7.
In this case not full thiol’s 8 conversion (~60%) appeared (scheme 7) (table 2, entry 1). With increasing the co-pyrolysis temperature to 450°C the formation of compound 7 increases, while at 550°C the compound 7 becomes the main product of the reaction (table 2, entry 3). Raising of co-pyrolysis temperature to 650°C leads to resinification and a decrease in yield of the reaction mixture (table 2, entry 4).
Scheme 7
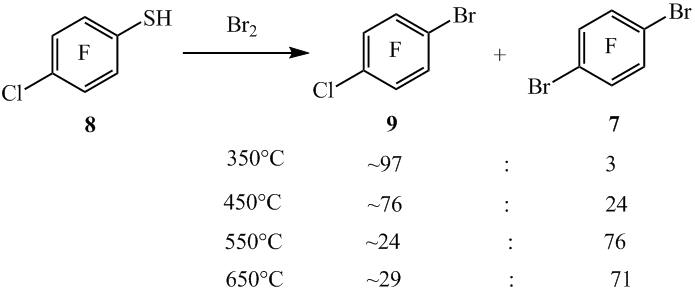
mole ratio of reaction products in reacting masses calculated from GLC data
The 4-bromoheptafluorotoluene (11) with the high yield was prepared from the 4-trifluoromethyl-2,3,5,6-tetrafluorobenzenethiol (10) and bromine in an ampule (scheme 8).
Scheme 8
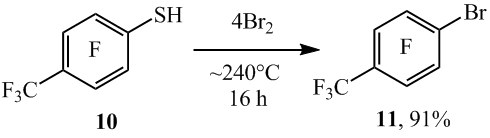
Similar from 5-nonafluoroindanethiol (12) and 6-bromooctafluoroindane-5-thiol (13) with the high yields there were synthesized 5-bromononafluoroindane (14) and 5,6- dibromooctafluoroindane (15) respectively (scheme 9).
Scheme 9
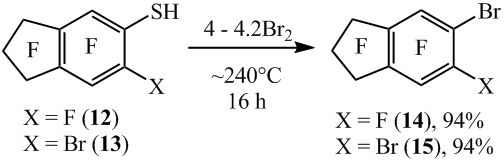
It also should be underlined that the compound 15 can be produced in a flowing reactor from the thiol 13 and Br2 with the yield of 85% (scheme 10).
Scheme 10
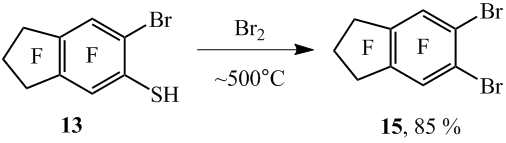
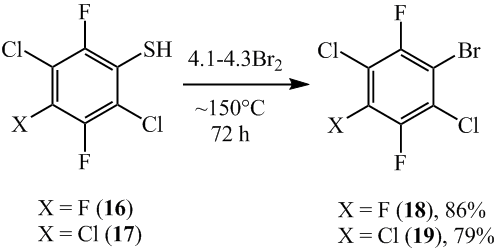
Varying the reaction time and temperature in ampules makes it possible to synthesize polyfluoroarenes, that at ortho-position contain chlorine and bromine atoms. In such a way, 1-bromo-2,5-dichloro-3,4,6-trifluorobenzene (18) and 1-bromo-2,4,5-trichloro-3,6-difluorobenzene (19) were produced by heating with bromine 2,5-dichloro-3,4,6-trifluorobenzenethiol (16) and 2,4,5-trichloro-3,6-difluorobenzenethiol (17) (scheme 11). By doing so, according to 19F NMR, GC-MS and GLC data, difluoro-derivants in small amounts appeared as co-products.
Scheme11
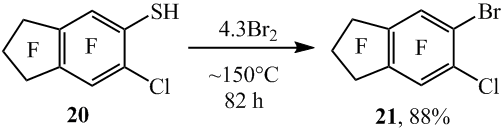
Similar when by brominating 6-chlorooctafluoroindane-5-thiol (20) 5-bromo-6-chlorooctafluoroindane (21) was produced (scheme 12), at the same time the formation of the compound 15, according to 19F NMR, GC-MS and GLC data, was practically not observed.
Scheme12
The substitution of the thiol group by bromine atom in polyfluoroarenethiols probably proceeds via homolytic mechanism similar to the substitution of the thiol group by chlorine atom in the co-pyrolysis of polyfluoroarenethiols with chlorine [2]. Polyfluorosulfenylbromides [6] and bis(polyfluoroarene)disulfides might appear as the intermediate products in the reaction of polyfluoroarenethioles with bromine. By analogy with the transformation of disulfide 3 under the action of Cl2 into pentafluorobenzosulfenylchloride [2], probably the transformation of bis(polyfluoroarene)disulfides under the action of bromine into the intermediate products (polyfluoroarenesylfenylbromides) on the way to bromopolyfluoroarene is possible. For example: the interaction of pentafluorobenzenesulfenylbromide (22) with the bromine atom with the formation of intermediate radical σ-complex (A) and following elimination of sulfur-containing function probably leads to the arene 2 appearance (scheme 13).
.
Scheme13
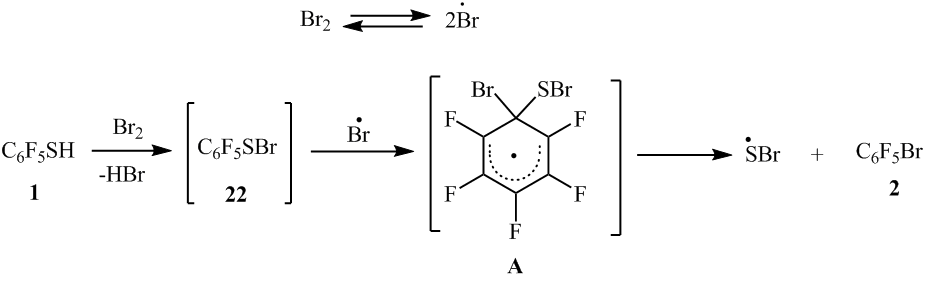
In the chloropolyfluoroarenethiols with Br2 reactions the substitution of the chlorine atoms by bromine seems to proceed via homolytic mechanism (scheme 14). When this happens, the substitution of the thiol group by bromine proceeds easier then substitution of chlorine by bromine.
Scheme14
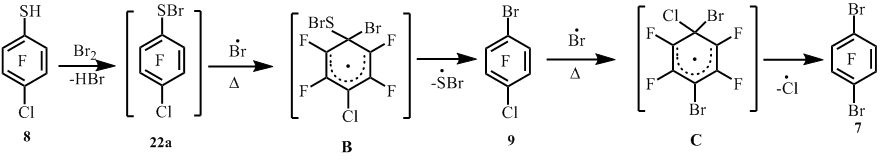
Probably, in the radical σ-complex (B) С-S bond is weaker than C-Br (bond energies С-S, E=52 kcal/mole, C-Br, E=58 kcal/mole [7]) that leads to elimination of the sulfur-containing function. In case of radical σ-complex (C) formation, for stronger C-Cl bond (E=93,8 kcal/mole [7]) substitution, the higher temperature (450-550°C) is required. Probably, in the compound 8 with bromine reaction the radical σ-complex (B) step formation is the rate-limiting step for sulfenyl bromide 22a into the compound 9 transformation under the bromine atom action, while in case of radical σ-complex (C) formation, the rate-limiting step will be the second with chlorine atom elimination (scheme 14).
To some extent, probably, the substitution of chlorine atom by bromine in the compound 8, followed by substitution of the thiol group by bromine atom in the intermediate thiol 6, can’t be excluded.
For the thiol 1 with PBr5 reaction, taking into account that in crystalline state PBr5 has an ion structure [PBr4]+Br-[8], then when involving cation [PBr4]+, sulfenyl bromide 22 might be formed, which when interacts with thiol 1 transforms into disulfide 3 (scheme 15). Also, the formation of disulfide 3 from thiol 1 and PBr5 without sulfenyl bromide 22 participation probably can’t be excluded. Disulfide 3 under the PBr5 action could give sulfenyl bromide 22 similar to the conversion of this disulfide 3 into pentafluorosufenylchloride in the reaction with PCl5 [4].
Scheme15
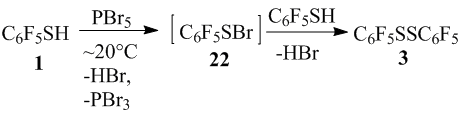
It is known that PBr5 boils and decomposes at 106°C [9] and in this way it is a bromine source. According to the assessment, that we have made by analogy with the same for PCl5 [10], the axial bond P-Br energy in the trigonal bipyramidal structure of PBr5 [11] is equal to 28 kcal/mole [10], whereas Br-Br bond energy is a higher measure (45.4 kcal/mole [7]). According to this, it’s possible to assume that initially PBr5 is decomposed with formation of bromine atom and • PBr4 radical (scheme 16, formula 1). • PBr4 radical also can be decomposed with the formation of PBr3 and • Br (formula 2). Further reactions, sulfenyl bromide 22 with bromine atom for example, probably gives radical σ-complex (A) [2], and at its decomposition the compound 2 appears (formula 5). • SBr radical that is eliminated at σ-complex (A) decomposition, if reacting with PBr3, then the phosphoranyl radical (D) might appear. The latter at β-decomposition gives thiophosphoryl bromide 23, that was fixed by us using 31Р NMR spectrum and GC-MS. It’s known that the phosphoranyl radicals, despite their relative stability, may undergo β-decomposition, including the one with generation of S=P bond [12].
Scheme16
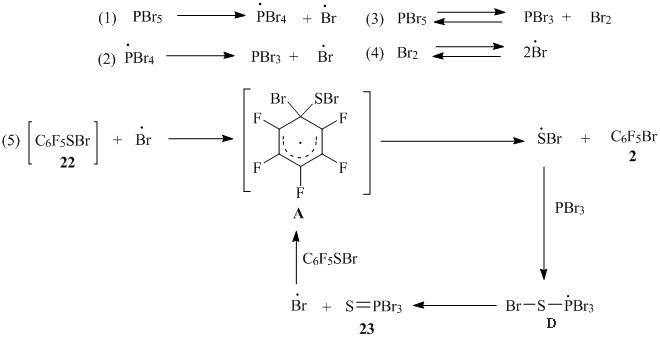
As far as the P-Br axial bond energy in PBr5 is less than Br-Br bond energy, it is possible to presume that PBr5 is a better source of atomic bromine than Br2. This fact probably explains why the thiol 1 with PBr5 reaction needs less time than with Br2.
Besides the formulas 1 and 2, on the scheme 16 there is shown the Br2 formation from PBr5 and the Br2 dissociation on the bromine atoms (formulas 3,4) by analogy with PCl5 transformation [4]. Such type of PBr5 decomposition shouldn’t be excluded.
P-Cl axial bond energy in trigonal bipyramid PCl5 [11] is equal to 34 kcal/mole [10], whereas Cl-Cl bond energy is a higher measure (57.2 kcal/mole [7]). According to this, there might took place the change of the compound 1 reactivity with respect to PCl5 [4] and Cl2 in favor of the process with PCl5. However, we did not study this question experimentally.
Experimental
The analytical and spectral studies were performed at the Joint Chemical Service Center, Siberian Branch, Russian Academy of Sciences.
31P, 19F and 1H NMR spectra were recorded on a Bruker AV–300 spectrometer (121 MHz for 31P , 282 MHz for 19F, 300 MHz for 1H) in CCl4 with CDCl3 additive. External standard for 31P NMR – H3PO4. For 19F and 1H NMR spectra internal standards – C6F6 and HMDS (0.04 ppm from TMS) respectively. The positive values of chemical shifts correspond to the signals downfield shift. IR - spectra were prepared on a Bruker Vector 22 IR. UV - spectra were recorded on a Hewlett Packard 8453 UV and Cary 5000. Molecular weights and elemental compositions were determined from the high-resolution mass spectra which were obtained using a DFS instrument (electron impact, 70 eV). A chromatograph НP 5890 with a mass-selective detector HP 5971 and a chromatograph Agilent 6890N with a system Agilent 5973N were used for GC–MS. The ionizing electron energy was 70 eV. Separation of substances was carried out on a column HP-5, 30 m x 0.25 mm x 0.25 μm, helium was used as a carrier gas with flow rate 1 mL/min, column temperature 50 - 280°C, ion source temperature 173°C. GLC-analysis was carried out on a chromatograph HP 5890 with a column HP-5, 30 m × 0.52 mm/2.6 μm and a thermal conductivity detector.
The starting thiols were prepared using a procedure given in [13].
A solution of KSH in ethylene glycol (~4.1 mol/l) was prepared by blowing hydrogen sulphide in the solution 2 mole КОН in 330 ml of in ethylene glycol till the mass increased on 64gr.
6-bromooctafluoroindan-5-thiol (13) preparation. Indan (14) solution 50.35 g. (140.26 mmole) in 100 ml of dimethyl carbinol on cooling by water with ice and while mixing during 1.5 hours was being added 71 ml of KSH solution in ethylene glycol, at no more than 5°C. Further, the reaction mixture was being stirred during 2.5 hours at 0-1°C. Then the reaction mixture was poured into a mixture of concentrated hydrochloric acid (350 ml) and ice (500 g). The residuum was separated, dried over CaCl2. The dry product mass – 48.95g. This was analyzed by 19F, 1H NMR and GLC methods. The content of the compound 13 by GLC – 98% (the yield – 91%). M.p. - 32-33.5°C (pentan). 19F NMR spectrum (δ, ppm): 31.9 quintet (2-CF2, J2-CF2–1,3-CF2 4.5 Hz), 51.2 dt (F4, JF4-F7 21 Hz, JF4–3-CF2 7.5 Hz), 53.7 broad multiplet (1(3)-CF2), 53.9 dt (F7, JF7-F4 21 Hz, JF7–1-CF2 7.5 Hz), 54.7 broad multiplet (1(3)-CF2). 1H NMR (δ, ppm): 4.56 s. UV-spectrum (hexane), λmax, nm (lgε,): 223 (4.19), 262 (3.82), 288 (3.18), 297 (3.21). IR- spectrum (KBr), ν, sm-1: 2587, 2568, 1627, 1455, 1298, 1247, 1204, 1154, 1096, 1060, 955, 944, 869, 804, 576, 543. Found, %: C 28.84; H 0.24; F 40.71; S 8.76; Br 21.25. M+ 371.8854. C9HBrF8S. Calculated, %:C, 28.98; H, 0.27; F, 40.74; S, 8.60; Br, 21.42. М+ 371.8849.
Reactions of thiol 1 with PBr5. 2.27 g. (11.34 mmole) of thiol 1 was placed into an ampoule and there was portioned added 10.13 g. (23.53 mmole) of PBr5. When gas isolation finished the ampoule was sealed up. Then the ampoule was placed in a metal sheath and was being heated at 218-220°C during 5 hours. Following of the end of the reaction the ampoule was being cooled with liquid nitrogen and opened. There was obtained 11.49 g of reacting masses, that according to GC–MS contains 46% of the compound 2 and 50% of Br3PS 23. Then content was transferred under water with ice (~60g) sheet into a flack. The mixture was being mixed by magnetic stir bar during 2 hours for the purpose of phosphorus compounds hydrolysis, then it was alkalized by Na2CO3 and was being mixed for 2 hours and it was steam distilled . The product (2.44g.) was separated, dried over CaCl2 and analyzed by 19F NMR and GLC methods. This product contents compound 2 - 98.3 % (according to GLC) and disulfide 3 – 0.5% (according to GLC).
Identification of compound 23 was carried out using 31P NMR (δ, ppm: - 115.3 p [14]) and GC-MS. The total mass-spectrum of the compound 23 is: 306 (15.1), 304 (42.3), 302 (42.2), 300 (14.6; Br3PS), 225 (52.8), 223 (100), 221 (51.0; Br2PS), 193 (3.7), 191 (7.4), 189 (3.7; Br2P), 112 (6.4), 110 (6.5; BrP), 81 (6.6), 79 (6.7; Br), 63 (26.0; PS), 32 (1.5; S), 31 (3.0; P).
By analogy, from the 2.12 g. (10.59 mmole) of thiol 1 and 89.14 g. (21.23 mmole) of PBr5 (at 218-220°C, 8 hours) this was prepared 2.21 of reacting mass with 99.1% content of compound 2 according to GLC, yield 84%.
The compound 3 (according to 19F NMR) without the formation of other polyfluoroarenes was prepared when 0.09 g. (0.45 mmole) of the compound 1 was mixed with 0.39 g. (0.91 mmole) PBr5 at room temperature.
Reaction of thiol 1 with PBr3 + Br2. From the 2.09 g. (10.44 mmole) of the compound 1, 5.68 g. (20.98 mmole) of PBr3 and 3.45 g. (21.59 mmole) of Br2 (at 218-220°C, 5 hours) this was prepared 2.25 g of reacting mass with 93.1% content of compound 2 (according to GLC) and with 4.4 % content of disulfide 3 (according to GLC).
Reaction of thiol 1 with PBr3. By heating 2.09 g. (10.44 mmole) of the thiol 1 and 5.77 g. (21.32 mmole) of PBr3 (at 218-220°C, 5 hours) this was prepared 1.91 of reacting mass, that contains the starting thiol 1 (60.1% according to GLC) and disulfide 3 (37.0% according to GLC).
Reaction of thiol 1 with Br2. 0.17 g. (0.85 mmole) of the compound 1 was placed into an ampoule and there were portioned added 0.61 g. (3.80 mmole) of bromine. When gas isolation finished the ampoule was sealed up. Then the ampoule was placed in a metal sheath and was being heated at ~220°C during 8 hours. Following of the end of the reaction the ampoule was being cooled, opened and the content of ampoule poured into water. Surplus of bromine was deleted using sodium sulphite, then it was extracted using ~2 ml of dichloromethane and analyzed by 19F NMR (2:3 ~84:16 according to 19F NMR data).
The general procedure for bromofluoroarenes synthesis. Polyfluoroarenethiol was placed into an ampoule and there were portioned added bromine. When gas isolation finished the ampoule was sealed up and was placed in a metal sheath and then was being heated. Following of the end of the reaction the ampoule was being cooled with liquid nitrogen, opened and then content was transferred into a flack under the water sheet (80-100 ml). Surplus of bromine was deleted using sodium sulphite, then the reacting mass was steam distilled. Surplus of bromine was deleted using sodium sulphite, then the reacting mass was steam distilled. The product was separated, dried over CaCl2 and analyzed by 19F NMR and GLC methods. By analogy the reaction of disulfide 3 with bromine was made. The results are shown in the Table 1.
The formation of the compounds 2,3,4,5,7,11 and 14 is proved by 19F NMR spectra [2,3,15].
Table 1.
|
# Entry |
Compound, g (mmole) |
Bromine, g (mmole) |
Molar ratio of thiol to Br2 |
Temperature, °С |
Time, hours |
Mixture yield, g |
Content (yield) of product by GLC, % |
|
1 |
1, 2.01 (10.04) |
3.31 (20.71) |
2.06 |
218-220 |
5 |
2.05 |
2, 52.7a (44) |
|
2 |
1, 2.05 (10.24) |
3.39 (21.21) |
2.07 |
218-220 |
8 |
2.11 |
2, 72.7b (61) |
|
3 |
1, 2.02 (10.09) |
3.32 (20.77) |
2.06 |
218-220 |
16 |
2.15 |
2, 91.5c (79) |
|
4 |
1, 2.10 (10.49) |
3.46 (21.65) |
2.06 |
218-220 |
22 |
2.32 |
2, 99.7 (89) |
|
5 |
1, 2.08(10.39) |
5.10 (31.91) |
3.07 |
218-220 |
16 |
2.34 |
2, 99.8 (91) |
|
6 |
1, 2.89 (14.44) |
9.51 (59.51) |
4.12 |
220-222 |
16 |
3.34 |
2, 99.9 (94) |
|
7 |
1, 2.76 (13.79) |
9.08 (56.82) |
4.12 |
250-255 |
16 |
3.18 |
2, 96.5d (90) |
|
8 |
3, 3.11(7.81) |
5.11 (31.98) |
4.09 |
219-221 |
16 |
3.65 |
2, 99.8 (94) |
|
9 |
4, 2.76 (15.15) |
9.91 (62.01) |
4.09 |
218-220 |
16 |
3.21 |
5, 99.0 (92) |
|
10 |
6, 2.52 (9.65) |
6.30 (39.42) |
4.08 |
220-222 |
16 |
2.68 |
7, 98.7 (89) |
|
11 |
8, 3.49 (16.11) |
11.2 (69.83) |
4.33 |
201-203 |
12 |
4.00 |
9, 91.9e (87) |
|
12 |
8, 3.46 (15.98) |
10.4 (65.02) |
4.07 |
185-187 |
16 |
3.41 |
9, 98.3f (80) |
|
13 |
10, 3.33 (13.31) |
8.53 (53.38) |
4.01 |
238-240 |
16 |
3.61 |
11, 99.8 (91) |
|
14 |
12, 2.79 (8.94) |
6.02 (37.67) |
4.21 |
238-240 |
16 |
3.05 |
14, 98.5 (94) |
|
15 |
13, 2.73 (7.32) |
4.73 (29.60) |
4.04 |
238-240 |
16 |
2.90 |
15, 99.7 (94) |
|
16 |
16, 7.31 (31.37) |
20.09 (125.71) |
4.29 |
149-151 |
72 |
8.09 |
18, 93.5j (86) |
|
17 |
17, 3.54 (14.19) |
9.23 (57.57) |
4.07 |
149-151 |
72 |
3.63 |
19, 91.8h (79) |
|
18 |
20, 3.74 (11.38) |
7.79 (48.75) |
4.28 |
150-152 |
82 |
3.81 |
21, 98.3i (88) |
aThe content of disulfide 3 in the reaction mixture is equal to 46.7% according
to GLC.
b The content of disulfide 3 in the reaction mixture is equal
to 27.0% according to GLC.
c The content of disulfide 3 in the reaction
mixture is equal to 7.9% according to GLC.
dAccording to GC–MS the reaction mixture
contents 3.4% of polybromopolyfluorobenzenes (GLC).
e According to GC–MS the reaction
mixture contents 8% of an arene 8 (GLC).
f According to GC–MS the
reaction mixture contents 1.4% of arene 8 (GLC).
j According to
GC–MS the reaction mixture contents 2.5% of dibromotrifluorochlorobenzenes (GLC).
h According
to GC–MS the reaction mixture contents 6.9% of dibromodifluorodichlorobenzenes (GLC).
i According
to GC–MS the reaction mixture contents 0.3% of indane 15 (GLC).
1-Bromo-4-chloro-2,3,5,6-tetrafluorobenzene (9) (98.3% according to GLC data). M.p. 55-57ºC. 19F NMR spectrum, δ, ppm: 22.6 m (F3,5), 30.3 m (F2,6). UV- spectrum (hexane), λmax, nm (lgε,): 222 (4.00). IR- spectrum (KBr), ν, cm‑1: 1742, 1634, 1493, 1462, 995, 964, 808, 594. Found, %: C 27.20; Br 30.50; Cl 13.30; F 29.17. M+ 261.8801 C6BrClF4. Calculated, %: C 27.36; Br 30.33; Cl 13.46; F 28.85. M+ 261.8803.
5,6 - Dibromooctafluoroindane (15). (99.7% according to GLC data). M.p. 37.5-38.5°C (pentan). 19F NMR spectrum, δ, ppm: 31.7 quintet (2-CF2, J2-CF2–1,3-CF2 ~ 4 Hz), 53.7 m (1(3)-CF2), 57.9 m (F4,7). UV- spectrum (hexane), λmax, nm (lgε,): 221 (3.79), 238 (3.64), 243 (3.57, shoulder), 280 (3.03), 288 (3.14). IR- spectrum (KBr), ν, cm‑1: 1724, 1624, 1607, 1460, 1439, 1398, 1331, 1290, 1246, 1221, 1205, 1159, 1096, 1051, 949, 874, 800, 689, 671, 575, 536. Found, %: С 26.09; F 36.37; Br 38.08. M+ 417.8230. C9Br2F8. Calculated, %:C, 25.74; F, 36.20; Br 38.06. M+ 417.8234.
1-Bromo-2,5-dichloro-3,4,6-trifluorobenzene (18). (98.3% according to GLC data). Distillation under vacuum (~12 mm Hg) of 4.74 g. of the mixture there were prepared 2.64 g. fraction with b.p. 94-95°C and purity 98.3% and 1.27 g. fraction with b.p. ~95°C and purity 95.1% according to GLC data. 19F NMR spectrum, δ, ppm: 27.6 dd (F3, JF3-F4 21, JF3-F6 9.5 Hz), 28.9 d (F4, JF4-F3 21 Hz), 57.0 d (F6, JF6-F3 9.5 Hz).. UV- spectrum (hexane), λmax, nm (lgε,): 225 (4.06), 280 (2.85). IR- spectrum (film), ν, cm‑1: 1597, 1468, 1441, 1354, 1308, 1076, 964, 874, 748, 700, 638. Found, %: С 25.91; Br 28.50; Cl 25.17; F 20.26. M+ 277.8508 C6Cl2BrF3. Calculated, %: C 25.75; Br 28.55; Cl 25.34; F 20.36. M+ 277.8507.
1-Bromo-2,4,5-trichloro-3,6-difluorobenzene (19). (96.9% according to GLC data). Distillation under vacuum (~12 mm Hg.) of 2.69 g. of the mixture there were prepared 1.20 g. fraction with b.p. ~127°C and purity 96.9% and 0.94 g. fraction with b.p. 127-128°C and purity 96.5% according to GLC data. M.p. 94-96ºC. 19F NMR spectrum, δ, ppm: 52.5 d (F3, JF3-F6 11 Hz), 60.4 d (F6, JF6-F3 11 Hz). UV- spectrum (hexane). λmax, nm (lgε,): 229 (4.12), 232 (4.13), 282 (3.26, shoulder), 290 (3.35). IR- spectrum (KBr), ν, cm‑1 1435, 1387, 1287, 1252, 901, 881, 860, 698, 677, 631. Found, %: С 24.50; Br 27.00; Cl 35.90; F 13.03. M+ 293.8210 C6Cl3BrF2. Calculated, %: C 24.32; Br 26.97; Cl 35.89; F 12.82. M+ 293.8212.
5-Bromo-6-chlorooctafluoroindan (21). (98.3% according to GLC data). 19F NMR spectrum, δ, ppm: 31.8 qiuntet (2-CF2, J2-CF2–(1)3-CF2 ~4.5 Hz), 48.5 dt (F7, JF7-F4 19.5 Hz, J F7–1-CF2 7.5 Hz), 53.8 and 54.6 broad multiplets (1(3)-CF2), 56.7 dt (F4, JF4-F7 19.5 Hz, JF4–3-CF2 7.5 Hz). UV- spectrum (hexane), λmax, nm (lgε,): 234 (3.93), 279 (3.34), 287 (3.42). IR- spectrum (film), ν, cm-1:, 1612, 1464, 1441, 1408, 1315, 1302, 1248, 1207, 1157, 1099, 1059, 966, 949, 880, 818, 702, 673, 592, 579, 546. Found, %: С 28.36; Br 21.50; Cl 9.90; F 40.44. M+ 373.8732. C9BrClF8. Calculated, %: C 28.79; Br 21.28; Cl 9.44; F 40.48. М+ 373.8739.
The co-pyrolysis reactions of polyfluoroarenethiols in a flow system
The co-pyrolysis reaction of thiol 8 with Br2 was made in a quartz tube (reactor with the sizes 400x20mm), that was being heated in a electric tube heater. Before starting, the system was blowed off with argon. The supplement of bromine and thiol 8 into the reactor was carried out synchronously from the separate dropping funnels in the flowing argon (~3 l/hour). Before starting the thiol 8 was melt. The products of bromination were collected in a receiving flask cooled with ice water. Afterwards the temperature of the reaction mixture was adjusted to room temperature, and the reaction mixture was treated with a sodium sulphite solution to remove the bromine and then it was steam distilled. The resulting solid product was separated from the water and dried over CaCl2 and analyzed by 19F NMR and GLC methods. The results are shown in the table 2.
Тable 2.
|
# Entry |
Compound 8, g (mmole) |
Bromine, g (mmole) |
Molar ratio of thiol to Br2 |
T, °С |
The yield of mixture, g |
Content (yield) of the product 9 by GLC, % |
Content (yield) of product 7 by GLC, % |
|
1 |
43.50 (200.85) |
88.10 (551.54) |
2.75 |
350 |
22.83A |
92.7 (40) |
3.8 (1) |
|
2 |
4.07 (18.79) |
8.77 (54.81) |
2.92 |
450 |
4.24 |
71.6 (61) |
26.2 (19) |
|
3 |
2.75 (12.70) |
9.36 (58.50) |
4.61 |
550 |
2.95 |
21.0 (18) |
77.7 (58) |
|
4 |
3.04 (14.04) |
13.80 (86.60) |
6.17 |
650 |
2.94 |
22.9 (18) |
64.1 (44) |
А The distillation residue was extracted with CHCl3 3×20 ml, and after boiling – down on a rotary evaporator there was prepared 31.14 g. with 56% content of thiol 8 (according to GLC data).
5,6 - Dibromooctafluoroindane (15). The reaction was made in a flow reactor by analogy with co-pyrolysis of thiol 8 with bromine. From 9.70 g. (26.00 mmole) of thiol 13 and 18.38g. (114.88 mmole) of Br2 at ~500°C there was prepared 9.90 g. of product. This product content 98% of the compound 15 according to GLC, with the yield – 85%.
Acknowledgement
This research was financially supported by the Russian Foundation for Basic Research (RFBR) (project No 15-03-08869a).
References
- Brooke G.M. The preparation and properties of polyfluoroaromatic and heteroaromatic compounds. J. Fluorine Chemistry. 1997. V. 86. № 1. P. 1–76.
- Platonov V.E., Maksimov A.M., Nikul’shin P.V., Dvornikova K.V. Organofluorine sulfur-containing compounds: V. Joint pyrolysis with chlorine or bromine of polyfluoroarenethioles, polyfluorohetarenethioles, and their derivatives Russ J. Org. Chem. 2005. V. 41. № 11. P. 1647-1653.
- Platonov V.E., Nikulshin P.V., Maximov A.M. Substitution of thiol groups in polyfluoroarenethiols for chlorine and bromine atoms. Production of chloro- and bromopolyfluoroarenes. Fluorine Notes. 2010. Vol. 1. /contents/history/2010/1_2010/letters/index.html.
- Nikul’shin P.V., Maksimov A.M., Platonov V.E. Synthesis of Chloropolyfluoroarenes from Polyfluoroarenethiols and PCl5. Russ J. Org. Chem. 2016. V. 52. № 2. P. 200-205.
- Antonucci J., Wall L. High-Temperature Reactions of Hexafluorobenzene. J. Res. Nat. Bur. Stand. 1966. V. 70a. № 3. P. 473-480.
- Neil R.J., Peach M.E., Spinney H.G., Pentafluorobenzenesulfenyl Halides and Pseudohalides. Inorg. Nucl. Chem. Lett. 1970. V. 6. № 5. P. 509–510.
- Energii razryva khimicheskih svyazej. Potencialy ionizacii i srodstvo k elektronu. Red. V.N.Kondrat'ev. M. Nauka. 1974.
- Cotton F.A., Wilkinson G. Advanced Inorganic Chemistry. N.-Y.: Intersci. Publ., a Division of J.Wiley&Sons Inc., 1966.
- Remy H. Lenrbuch der Anorganischen Chemie. Band I. Akademische Verlagsgesellschaft Geest & Portig K.-G. Leipzig. 1960.
- Hartley S.B., Holmes W.S., Jacques J.K., Mole M.F., McCoubrey J.C. Thermochemical properties of phosphorus compounds. Quarterly reviews. 1963. V. 17. № 2. P. 204–223.
- Nekrasov B.V. Kurs obshchej khimii, M. Gosudarstvennoe nauchno-tekhnicheskoe izdatel'stvo khimicheskoj literatury. 1960. S. 396.
- Smith, L.J.H., Comprehensive Organic Chemistry. The Synthesis and Reactions of Organic Compounds, Barton, D. and Ollis, W.D., Eds., Oxford: Pergamon Press, 1979. V. 2. P. 1158.
- Maksimov A.M., Platonov V.E. Reactions of some polyfluoroaromatic compounds with potassium hydrosulfide. Fluorine Notes. 1999. Vol. 4. /contents/history/1999/4_1999/letters/index.html.
- Muller N., Lauterbur P.C., Goldenson J. Nuclear Magnetic Resonance Spectra of Phosphorus Compounds. J. Am. Chem. Soc. 1956. V. 78. № 15. P. 3557–3561.
- Pushkina L.N., Stepanov A.P., Zhukov V.S., Naumov A.D. Spektry NMR 19F zameshchyonnyh pentaftorbenzolov. ZhOrKh. 1972. T. 8. Vyp. 3. S. 586-597.
Recommended for publication by Prof. V. E. Platonov
Fluorine Notes, 2016, 106, 1-2
