Received: November, 2015
DOI 10.17677/fn20714807.2016.02.03
Fluorine Notes, 2016, 105, 5-6
FLUORINE INFLUENCE ON PHARMACEUTICAL ACTIVITY OF TRIUROCYL DERIVATIVES
Е.S. Titova1, P.M. Vassiliev2, А.I. Rakhimov1, V.V. Vorfolomeeva2, L.R. Yanalieva2
1Volgograd State Technical University, 400131, Russia, Volgograd, Lenin prospect,
28
e-mail: titova051@rambler.ru
2Volgograd State Medical University, Russia, 400131, Volgograd, sq. Pavshikh Borzhov,
1,
e-mail: pvassiliev@mail.ru
Abstract: Consensual forecast of pharmaceutical activity of 5-[4-(N,N-dimethylamino]-2-trioxohydropyrimidine-4,6-(1Н,5Н)-dione and its fluorine derivatives is made in the system PASS, IT «Microcosm», by doking method in AutoDock Vina and by quantum chemical QSAR-simulation method in order to discover a new perspective antidiabetic agents.
Keywords:5-[4-(N,N-dimethylamino)phenylmethylene]-2-trioxodihydriopyrimidine-4,6-(1Н,5Н)-dione and its fluorine derivaties, inhibitors glycogen phosphorylases, inhibitors α-glycocidases, inhibitors of nonenzymatic glycation of proteins, consensual forecast.
1. Introduction
Triurocyl derivatives have a wide range of biological activity [1,2],that is due to the properties of their electronic structure [3-8].
A non-insulin dependent diabetes (TYPE 2) has the form of the group of heterogeneous distortion of carbohydrate metabolism disorder, which one is due to insulin resistance, insulin secretory function of pancreatic disorder and the glucose escape in the liver. The prevalence of diabetes has reached epidemic proportion as in the world and in Russia at the present time. [9].
A hepatic form of glycogen phosphorylase (PYGL) is mostly involved into glucose homeostatic in the body and it is the object for therapeutic intervention at TYPE 2 [10]. The decreasing activity of PYGL is significantly reduced hyperglycosemia, which one does not lead to hyperglycosemia [11]. Therefore a search of antidiabetic drugs among PYGL inhibitors is of immediate interest.
α-glycocidase (MGAM) is settled in small bowel and catalyzing hydrolytic degradation of di-, oligo- and polysaccharides. The inhibitors MGAM blocks this process slowing down the absorption of monosaccharides, as a result a postprandial blood glucose is reduced (postprandial hyperglycosemia caused by eating) [12].
A nonenzymatic glycation of proteins becomes more strong at diabetes due to chronic hyperglycaemia, which is caused to different difficult complications, such as nephroparthy, encephaloparthy and others. That is why the search of inhibitors for Maillard reaction (MRI) is perspective direction of a new type of antidiabetic drugs creation [13].
2. Experimental
As a new perspective antidiabetic agents we considered the functional derivatives of triurocyl: 5-[4-(N,N-dimethylamino)phenylmethylene]-2-tioxodihydropyrimidine-4,6-(1Н,5Н)-dione (I) and its fluorine derivatives, where is fluorine atom in о- and m-positions towards to N,N-(dimethylamino)-group (II) and (III) respectively.
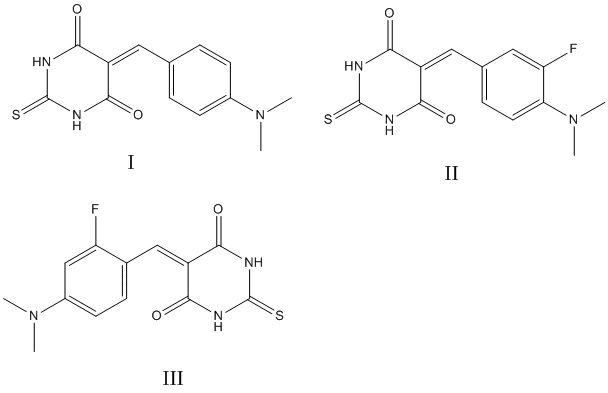
The effect of fluorine atom on the electron density of thiopyrimidine cycle differs in o - and m-positions. The conjugation of lone electronic pair of fluorine and nitrogen atoms with p-electronic system of aromatic rings is decreased along with the removal of fluoride from o - to m-position to N,N-(dimethylamino)-group. Negative inductive effect of the fluorine atom appears both in (II) and (III).
We made a consensual forecast of pharmaceutical activity of compounds (I) - (III) in the system PASS [14], in IT «Microcosm» [15, 16], by doking method in AutoDock Vina [17] and by quantum chemical QSAR-simulation method [18]. According to the forecast of investigated compounds it is expected the presence of activity as the inhibitors of non-enzymatic glycation of proteins (MRI), inhibitors glycogen phosphorylases (PYGL), inhibitors α-glycocidases (MGAM). The calculation date are provided in the table 1.
Table 1. The results of a consensual forecast of pharmaceutical activity of triurocyl derivatives.
|
Compound |
Activity |
Consensus type |
Estimated figure |
Value of estimated figure |
|
(I) |
MRI |
PC1QM |
Ind |
1.00 |
|
Fb |
0.4002 |
|||
|
Rank |
3.33 |
|||
|
PYGL |
PC1Dock |
Ind |
0.40 |
|
|
Fb |
0.1615 |
|||
|
Rank |
3.20 |
|||
|
MGAM |
PC2 |
Ind |
0.86 |
|
|
Fb |
0.3335 |
|||
|
Rank |
3.00 |
|||
|
(II) |
MRI |
PC1QM |
Ind |
1.00 |
|
Fb |
0.4002 |
|||
|
Rank |
3.33 |
|||
|
PYGL |
PC1Dock |
Ind |
0.30 |
|
|
Fb |
0.1293 |
|||
|
Rank |
2.70 |
|||
|
MGAM |
PC2 |
Ind |
0.86 |
|
|
Fb |
0.3335 |
|||
|
Rank |
3.00 |
|||
|
(III) |
MRI |
PC1QM |
Ind |
1.00 |
|
Fb |
0.4002 |
|||
|
Rank |
4.00 |
|||
|
PYGL |
PC1Dock |
Ind |
0.30 |
|
|
Fb |
0.1293 |
|||
|
Rank |
2.40 |
|||
|
MGAM |
PC2 |
Ind |
0.71 |
|
|
Fb |
0.2918 |
|||
|
Rank |
3.71 |
Reference.
PCK1QM – partial key consensus of the first
level: two positive forecast evaluations from three one, at normative positive evaluation
by quantum chemical QSAR-simulation method.
PCK1Dock – partial key consensus of the first level: two positive forecast evaluations from three one, at normative positive evaluation by doping method.
PC2 – partial key consensus of the second level: three positive forecast evaluations from four equal one.
Ind – average grade of perceptivity of activity presence (the most best value = 3, the worst value = 0); calculation method is described in [19, 20].
Fb – membership function to active compounds class (the best value = 1, the worst value = 0); calculation method is described in [21].
Rank – average evaluation grade of activity presence by all forecast methods, it is calculated for each activity in each consensus group (the most best value = 1, the worst value = 6).
As it is seen from the table 1,inhibiting ability of MRI and MGAM is not changing at transferring from the structure (I) to the structure (II). However, during fluorine atom introduction into m-position N,N-(dimethylamino)-group it is observed Rank parameter degradation for MRI activity at 0.67. It points to possible decreasing of reactivity of compound (III) in Maillard reaction. Rank parameter for a ability of inhibit α-glucocidase (MGAM) is also decreased. The decreasing consists of 0,71. Simultaneously at fluorine atom introduction is going on the improvement of Rank parameter for PYGL activity for structure (II) at 0,5 in compassion with (I) and for structure (III) at 0,8 in compression with the structure (I). At the same time Ind and Fb parameterы are worse a little bit.
3. Conclusion
It is established that fluorine atom introduction into m- and o-positions to N,N-(dimethylamino)-group in 5-[4-(N,N-dimethylamino)phenylmethylene]-2-trioxodihydropyrimidine-4,6-(1Н,5Н)-dione decreases the occurrence probability of compounds inhibiting Maillard reaction and inhibiting α-glycocidase activities. But it increases the occurrence probability of the presence of inhibiting glycogen phosphorylase activity. The most signified influence is observed at fluorine introduction in m-position.
This study was supported by Russian Scientific Fund (project # 14-25-00139).
References
- E.S. Titova, A.I. Rakhimov, V.A. Babkin, A.V. Ignatov, G.E. Zaikov, O.V. Stoyanov, Eli M. Pearce. Computer screening of biological activity S-, and S, O-derivatives of 6-methyl-2-thiouracil // Bulletin of Kazan Technological University / - 2014. - V. 17, Iss. 13. - P. 264-265.
- A.I. Rakhimov, E.S. Titova. Features of the synthesis of 2-alkyl(aryl-alkyl)-6-methylpyrimidin-4(3H)-ones and 2-alkyl(arylalkyl)thio-4-alkyl(arylalkyl) oxy-6-methylpyrimidine // Journal of Organic Chemistry / - 2007. – Iss. 1. - P. 92-98.
- A.I. Rakhimov, E.S. Titova, R.G. Fedunov, V.A. Babkin. Properties nucleophilic substitution of halogen in the alkyl and benzyl anions generated from 4-hydroxy-2-mercapto-6-methylpyrimidine // Chemistry of Heterocyclic Compounds / - 2008. - Iss. 6. - P. 874-883.
- A.I. Rakhimov, E.S. Titova. Electronic structure and properties of thiouracil // New Trends in Chemistry of Heterocyclic Compounds: mater. Intern. Conf. (Kislovodsk, May 3-8 2009) / - Kislovodsk, 2009. - P. 419.
- A.I. Rakhimov, E.S. Titova, R.G. Fedunov, V.A. Babkin, О.V. Stoyanov, G.E. Zaikov. Generation of S-, O-anion from 6-methyl-2-thio-, 2-alkyl(aralkyl)thiouracils in the synthesis of S-mono and S-, O-diderivatives // Bulletin of Kazan Technological University / - 2013. - V. 16, Iss. 5. - P. 23-29.
- A.I. Rakhimov, E.S. Titova, R.G. Fedunov, V.A. Babkin, O.V. Stoyanov, G.E. Zaikov. Quantum chemical calculations of thio- and oxy-anions generated from 2-mercapto-4-hydroxy-6-methylpyrimidine // Bulletin of Kazan Technological University / - 2014. - V. 17, Iss. 13. - P. 7-8.
- V.A. Babkin, D.S. Аndreev, E.S. Titova, I.Yu. Kameneva, А.I. Rakhimov. The geometric and electronic structure of fluorinated pyrimidine // Energy-saving in the construction industry. Organizational-economic and social problems in construction management: mater. scientific and engineering. Internet Conf. SF VolgSABU, June 1, 2010, Mikhailovka Volgograd region / Sebryakov branch of VolgSABU [et al.]. - Volgograd, 2010. - P. 131-148.
- V.A. Babkin, I.А. Korotkova, E.S. Titova, G.E. Zaikov, О.V. Stoyanov, D.S. Andreev. Quantum-chemical calculations of some pyrimidines by method MNDO // Bulletin of Kazan Technological University / - 2012. - V. 15, Iss. 21. - P. 7-9.
- I.I. Dedov, М.V. Shestakova, Yu.I. Suntsov. Diabetes in Russia: Problems and Solutions. - Moscow, 2008. - P. 3-6.
- L. Somsak, K. Czifrak, M. Toth et al. New inhibitors of glycogen phosphorylase as potential antidiabetic agents // Curr. Med. Chem. - 2008. - V.15. - Iss. 28. - P. 2933-2983.
- С. Wu, D. Okar, J. Kang, A.J. Lange Reduction of hepatic glucose production as a therapeutic target in the treatment of diabetes // Curr. Drug Targets Immune Endocr. Metabol. Disord. - 2005. V. 5. - Iss. 1. - P. 51-59.
- V.I. Pankiv. New therapeutic options control type 2 diabetes: the experience of the application of voglibose // International Journal of Endocrinology. - 2014. - Iss. 6(62). - P. 51-54.
- N.А. Ansari, Z. Rashid. Non-enzymatic glycation of proteins, from diabetes to cancer // Biomedical Chemistry. - 2010. - V. 5. - Iss. 2. P. 168-178.
- Filimonov D.А., Poroikov V.V. Forecast of spectrum of biological activity of organic compounds // Russian Сhem. J. (J. Of Russian chem. Society of D.I. Mendeleev). - 2006. - V. 50. - Iss. 2. - P. 66-75.
- Certificate of state registration of the computer program Iss. 2011618547. IT «Мikrosom» / Vassiliev P. M., Коchetkov А.N. (Russia). - Iss. 201616643; 02.09.2011 stated; eV. 31.10.2011; publ. 20.03.2012, Official Bulletin "Computer Programs for DB. TIMSS », Iss. 1(78), 2012, p. 209.
- Vassiliev P. M., Spasov A. A., Kosolapov V. A., Kucheryavenko A. F., Gurova N. A., Anisimova V. A. Consensus Drug Design Using IT Microcosm // Application of Computational Techniques in Pharmacy and Medicine / Eds. L. Gorb, V. Kuz’min, E. Muratov / Challenges and Advances in Computational Chemistry and Physics / Ed. J. Leszczynski. - Vol. 17. - Dordrecht (Netherlands): Springer Science + Business Media, 2014. - 550 p. - P. 369-431.
- Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading // J. Comp. Chem. - 2010. - V. 31. - Iss. 2. - P. 455-461.
- Vassiliev P. M., Spasov A. A., Kuznetsova V.А., Коchetkov А.N., Frantseva V.V., Yanalieva L.R. Quantum-chemical QSAR-model of inhibiting Maillard reaction // Abstracts XXII Russian National Congress "Man and Medicine" (Moscow, April 7-10. 2015). - Moscow, 2015. - P. 184.
- Frantseva V., Vassiliev P., Spasov A., Kuznetsova V., Solovieva O., Popov Yu., Lobasenko V., Korchagina T., Efremova O. Virtual and experimental screening of the Maillard reactions inhibitors in class of diphenyl oxide derivatives // 2nd Russian Conference on Medicinal Chemistry (MedChem-2015): Book of Abstracts (Novosibirsk, Russia, 5-10 July 2012). - Novosibirsk, 2015. - P. 180.
- Yanalieva L., Vassiliev P., Spasov A., Cheplyaeva N., Vorobiev E., Popov Yu., Lobasenko V., Shishkin E. and Mokhov V. Consensus screening of new inhibitors of glycogen phosphorylase in class of adamantane derivatives // 2nd Russian Conference on Medicinal Chemistry (MedChem-2015): Book of Abstracts (Novosibirsk, Russia, 5-10 July 2012). - Novosibirsk, 2015. - P. 311.
- Vassiliev P. Calculation of the Membership Function in the Consensus Prediction of the Pharmacological Activity of Chemical Compounds // 2nd Russian Conference on Medicinal Chemistry (MedChem-2015): Book of Abstracts (Novosibirsk, Russia, 5-10 July 2012). - Novosibirsk, 2015. - P. 302.
Recommended for publication by Prof. A. Rahimov
Fluorine Notes, 2016, 105, 5-6
