Received: November 2015
DOI 10.17677/fn20714807.2016.02.01
Fluorine Notes, 2016, 105, 1-2
A synthesis of a new fluoroorganosilicon compounds containing cyclobutane fragments and study of properties of obtained oligomers
Yarosh A.А., Sakharov А.М., Glazkov A.A.
N.D. Zelinsky Institute of Organic Chemistry Russian Academy of Science,
117991, Moscow,
Leninsky prospect, 47,
e-mail: yar@ioc.ac.ru,
as@zelinsky.ru
Abstract: At interation of perfluoroalkylvinyl ethers and butadiene there were synthesized a new polyfluorinated compounds – 1-vinyl-2-ω-cianoperfluoroalkoxi-2,3,3-trifluorocyclobutanes. It was established that addition of butadiene to perfluoroalkylvinyl ether was going on under "head to head". There were obtained corresponded chlorosilyl derivatives. There were obtained polyfluoroorganosiloxanes at hydrolysis of these chlorosilyl derivates. There were studies some properties of polyfluororganiloxanes.
Keywords: perfluoroalkyl vinyl ethers, butadiene. cycloaddition, hydrosilylation, polyfluororganosiloxane, oligomer properties.
Production of aliphatic polyfluorinated organo-silicon compounds is of a great scientific and applicative interest. The main methods of synthesis of organo-silicon compounds containing fluorine's atom in organic radical were summarized in the monographs of V.А. Ponomarenko and their stuff. [1,2].
This work was devoted to the synthesis of polyfluoroorganosilanes and siloxanes containing nitrile groups. Offered method of synthesis of fluoroorgano silicon compounds supposed two steps: the first one is polyfluorinated cyclobutane derivative production containing multiple bond, the second one is application of well known hydrosilylation reaction of unsaturated compound.
It is known that fluoro-containing alkenes could be added to nonfluorinated unsaturated compounds far easier then dimerized [3,4]. In the reaction of non-fluorinated dienes cycloaddition to fluoroalkenes, namely, to perfluoroalkylvinyl ether (PFAVE), it could be formed not only isomer (addition «head to head» or «head to tail»), but also their stereo isomers. The isolation and exact characterization of forming isomer is essentially complicated when they have a large molecular weight and high boiling point. That is why it seems appropriate to establish the principle of their formation and structure, low molecular weight analogues of these compounds.
In this work PFAVE was used as fluorinated dienes that contained nitrile group. The general method is described in the scheme below 1 [5,6]:
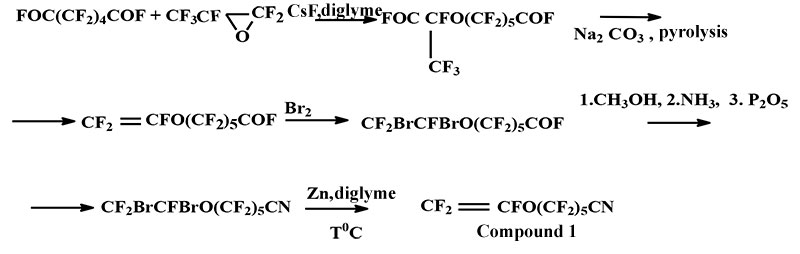
Scheme 1
Compound 2 was obtained analogically, scheme 2 [6]:

Scheme 2
A cycloadditon of PFAVE to butadiene could be done in two routes: «head to head» or «head to tail». 1Н and 19F NMR spectra of cyclodimerization products show that it could be realized in experiment conditions, if there would be only one type of addition, namely "head to head" with equal content of cis- and trans- isomers relatively to the cycle [4] (scheme 3):
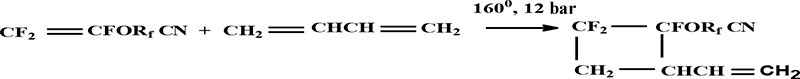
Rf = -(CF2)5 Compound 3 ; -(CF2)3OCF(CF3) Compound 4;
Scheme 3
It was carried out the separation of cis- and trans -isomers -vinyl-2-perfluoropropyloxy-2,3,3-trifluorocyclobutabe by HPLC (High-performance liquid chromatography) method for accurate assignment of signals in NMR spectra and description of the stereochemical structure of obtained products. As a result, we managed to isolate preparative pure isomers in order to identify their -19F NMR spectra. It was established that the peak with early retention time corresponded to cis-isomer, and with late one corresponded to – trans-isomer [8].
It is known that electric field's duopoly of neighboring bonds has a significant influence on chemical shift of 19F nucleus across the space, for example, so-called «ortho-effect» in fluorobenzenes. The size of this effect depends upon interatomic distance: as smaller interatomic distance between 1Н and 19F, resonance atoms 19F is observed in weak field [9]. Based on it, the signals in the field -120 ppm could be referred to fluorine atom of −CF− group in cis-isomer, and the signals in the field -140 ppm could be referred to the same group in trans-isomer.
As a result of hydrosilylation of compounds 3 and 4 there were obtained corresponded dichlorosilanes and oligosiloxanes (scheme 4):
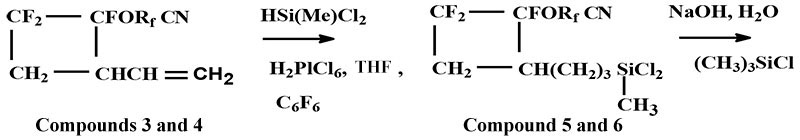
Rf is mentioned above in Scheme 3
Scheme 4
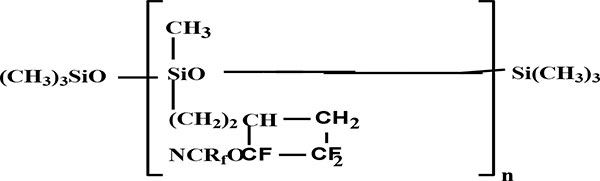
As a result of hydrolysis of methyldichlorosilane derivates there were synthesized oligomers with terminals silanol groups. The stabilization of last ones by trimethylchlorosilane gives a rise to oligomer formation with molecular weight 4500-5000 (ebullioscopy in C6F6). The temperature of such oligomers consists of 260°С at 10% loss of weight, it is a result of dehydrofluorination at heating [10].
A reaction control was made by NMR spectroscopy. There were appeared signals in the area (ppm): 3.80 (ОСН2); 1.02 (SiCH2); and 0.76 (SiCH3) in Proton magnetic resonance spectroscopy along with conversion increase. In the same time there were disappeared the signals in the area (ppm): 5.30 (=СН2) and 5.87 (=СН).
Obtained silanes and siloxanes could be used for creation of different composition on their basis, which one could have potentially useful properties in practice.
Experimental
19F and 1Н NMR spectra are registered on spectrometer “Bruker WN-250» with frequency 235 (external standard CFCl3) and 250 MHz (external standard TMS), accordingly. 29Si spectra were registered on spectrometer «Bruker-АС-200P» with frequency 39.76 MHz (external standard TMS). IR-spectra were registered on spectrometer «Specord IR-75». HPLC was made on liquid chromatographer LC21CP «Bruker», equipped with refractometric detector on semi-preparative silicagel column IBM (10.0x250х5), eluent – hexane, 2 ml/min. GLC-analysis was made on chromatographer LHM-8MD (column 1000×3,5 mm, carrier Chromatone 0.160-0.200 mm, treated by 5% Silicon SE-30). A starting chlorsilanes were supplied by Acros Organics, (purity not less than 96%). A catalyst was made under the following method: 1 g of H2PlCl6×Н2О was loaded into flask containing 100 ml of THF, preliminary distilled under СаН2, and obtained solution was kept in closed flask at room temperature within 168 hours.
A synthesis of compound 1 is described in [4]. The compound 2 was obtained by debromination in the presence of Zn-dust and metal iodine in diglime, similar to method [6]. Yield 87%, b.p.=105°С.
General method for fluorocyclobutanes preparation 3 and 4:
0.05 mole of PFAVE and 0.05-0.006 mole of butadiene were loaded into steel autoclave with volume 50 ml. An autoclave was heated up to 120° and kept at this temperature within 6-8 hours. In addition autogenic pressure was increased to 10-12 atm. After cooling and pressure releasing the content of autoclave was purified by rectification. The yield of compounds 3 and 4 consisted of 70-75%.
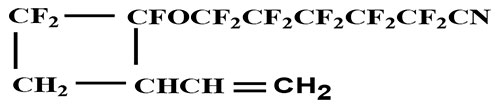
Compound 3
B.p.=71°С/10 torr. Elemental analysis: found, %: C 33.77; H 1.61; F 57.01. Calculated for C12H6F13NO, %: C 33.72; H 1.40; F 57.84.
1H NMR spectroscopy (δ, ppm): 5.80 m (1Н СН=); 5.30 m (2Н =СН); 3.30 m (1Н СН in the cycle); 2.80 m and 2.45 m (2Н СН2 in the cycle).
19F NMR spectrum (δ, ppm): two АВ-spectra: δА -77.7, δВ -85.9, JAB= 152 Hz; δА -80.1; δВ -84.9; JAB= 148 Hz; (2F OCF2 cis- and trans-isomers); two АВ-spectra: δА -107.5, δВ -110.0, JAB= 208 Hz; δА -108.2; δВ -212; JAB= 148 Hz; (2F CF2 in the cycle, cis- and trans-isomers); -105.3 m (2F CF2CN); -121.4 m (2F CF2CF2CN); -122.2-125.3 m (4 F -CF2CF2-); -121.0 m (cis); -138.9 m (trans) (1F CF-O).
IR spectrum, ν, cm-1: 2260 (CN).
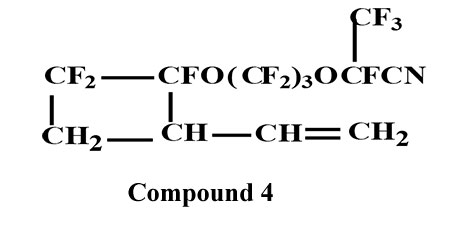
B.p.=80°С/16 torr. Elemental analysis: found, %: С 32.61; H 1.57; F 55.37. Calculated for C12H6F13NO2, %: C 32,52; H 1,36; F 55,34.
1H NMR spectroscopy (δ, ppm): 5.80 m (1Н СН=); 5.30 m (2Н =СН); 3.30 m (1Н СН in the cycle); 2.80 m and 2.45 m (2Н СН2 in the cycle).
19F NMR spectrum (δ, ppm): -78.1;78.9;81.5;82.0 (OCF2); 83.1 (CF3); 105.2; 109.2; 114.4; 118.0 (CF2 in the cycle); 120.0 141.3 (CF in the cycle, cis- and trans); 115.0 CF-CN); 128.6 128.9 (CF2CF2). IR-spectrum, ν, cm-1: 2260 (CN).
Hydrosilylation of fluoro-containing cyclobutane by methyldichlorosilane.
Into a four-neck flask equipped with stirring, dropping funnel, thermometer and reflux condenser was loaded 15.76 g (0.0355 mol) vinylcyclobutane 3. A flask was blown by argon. 0.5 ml 0.1N solution of H2PlCl6 in THF was added. Than 8.2 g (0.071 mol) of methyldichlorosilane was added at rapid mixing. The content of flask was heated to 60°С and kept within 2 hours. A reaction products were fractionated. It was obtained 16.1 g (80.8%) of compound 5. B.p.=90-91°С/1torr.
1H NMR spectroscopy (δ, ppm.): 0.63 (Si-CH3); 0.70 -1.10 (Si-CH2); 1.5-2.00 (Si-CH2 CH2); 2.20-2.70 (СН2-СН in the cycle). 19F NMR spectrum (δ, ppm): 78.1; -78.9; -81.5; -82.0 (OCF2) -83.1 (CF3); -105.3; -114.4; -118.0 (CF2 in the cycle); -120.0; -141 (CF – in the cycle); -115.0 (СFCN); -128.6; -128.9 (CF2CF2).
29Si, NMR spectrum (δ, ppm): 30.0. IR spectrum, ν, cm-1: 1000-1200 (СF); 1410 (CH in SiCH3); 2260 (CN); 2800-3000 (CH).
A compound 6 was obtained analogically, yield 66,7%. B.p. = 105-107°С/1torr. Chemical shifts in 1Н, 29Si NMR spectra of this compound were matched in a range of measurement accuracy in accordance with corresponded area of compound 6. 19F NMR spectrum (δ, ppm.): -77.2; -78.5; -82.2; -83.1 (OCF2); -105.4 (CF2CN); -109.4; -109.6; -114.2; -118.1 (CF2 in the cycle); -123.0; -124.4; -126.0 (CF2CF2 CF2); -119.9; -141.9 and -141.2 (CF in the cycle).
IR-spectrum, ν, cm-1: 2260 (CN).
References
- V.A. Ponomorenko, S.P. Krukovsky, A.Yu. Alybina. Fluorinated Heterochain Polymers. M. Nauka, p. 257, 1973.
- V.A. Ponomorenko, M.A. Ignatenko. Chemistry of Fluorosilicone Organic Compounds. M. Nauka, p. 191, 1979.
- J.D. Roberts, C.M. Sharts. Organic Reactions, 12, M. Mir, 1965, p.7.
- A.A. Glazkov, A.V. Ignatenko, S.P. Krukovsky, V.A. Ponomorenko. Izv. AN SSSR, ser. Khim., 1988, p. 2372 (Bull. of the Academy Sci., 1988, p.2137-2141).
- A.A. Glazkov, A.V. Ignatenko, S.P. Krukovsky, V.A. Ponomorenko. Izv. AN SSSR, ser. Khim., 1979, p. 2515 (Bull. of the Academy Sci., 1979, p.2327-2330).
- R. Sullivan. J.Org. Chem., 1969, v.34, p.1841.
- P.R. Resnick. US Patent 4474899, Chem. Abstr., 1984, v. 101, P 231608.
- A.A. Glazkov, V.M. Menshov, A.V. Ignatenko, S.P. Krukovsky, V.A. Ponomorenko. 6th Conference on Chemistry of Fluorosilicone Organic Compounds. Novosibirsk, 1990, abstract, p. 27.
- J.W. Emsly, J. Feeney, L.H. Suteliff. Progress in NMR Spectroscopy, 1971, v.7, p.3.
- A.A. Yarosh, A.A. Glazkov, L.I. Ignatischenko, A.M. Sakharov. 13th Andrianov’s Conference on Fluorosilicone Organic Compounds: Synthesis, Properties, Applications. Proceedings, Moscow, 2015, РО 91, p. 169
Recommended for publication by Prof. S. P. Krukovsky
Fluorine Notes, 2016, 105, 1-2
