Received: November 2015
DOI 10.17677/fn20714807.2016.01.01
Fluorine Notes, 2016, 104, 1-2
A synthesis of a new fluoroorganosilicon compounds and study of properties of obtained oligomers and polymers based on these oligomers.
Yarosh A.А., Sakharov А.М., Glazkov A.A.
N.D. Zelinsky Institute of Organic Chemistry Russian Academy of Science,
117991, Moscow, Leninsky
prospect, 47,
e-mail: yar@ioc.ac.ru, as@zelinsky.ru
Abstract: There were developed method of synthesis of a new fluoro organosilicon compounds based on hexafluoroacetone (HFA) and allyl bromide. As follows from the hydrosilylation of allyl ether cyanohydrin HFA there were synthesized a range of organosilicon intermediates - starting compounds for obtaining fluorocontaining oligosiloxanes, some of their properties were studied.
Keywords: Hexafluoroacetone, hexafluoroacetone cyanohydrin, allyl ether, sylylation, hydrolysis of chlorosilanes, fluoroorganosilicon oligomers.
The properties of polyfluoroorganosiloxanes are mainly defined by the structure of fluororganic radicals connected with silicon in polysiloxane chain. Such oligomers and polymers carry a range of unique properties: a high thermal and high chemical stability, especially in aggressive environments, a good greasy properties, and also a low surface energy providing high oil- and water repellence. A number of oligomers and polymers are produced in industrial scale at the present time [1, 2].
Oligomers and polymers containing fluoroorganic and organosilicon fragments as in main and side chains of molecules, however, they often carry a new and quite interesting properties, while do not produced in significant quantities. First of all it is related to the limited number of available starting fluoroorganosilicon compounds, also to the some difficulties of their synthesis.
In an article there are offered a new methods of synthesis of such compounds and provided some properties of oligomers and polymers obtained on their basis.
At interaction of hexafluoroacetone (HFA) with potassium cyanide in the presence of cesium fluoride in diglyme at −30°С it was obtained HFA cyanohydrin potassium salt and at its interaction with allyl bromide is formed correspondent HFA cyanohydrin allyl bromide (compound 1) under scheme 1[3]:
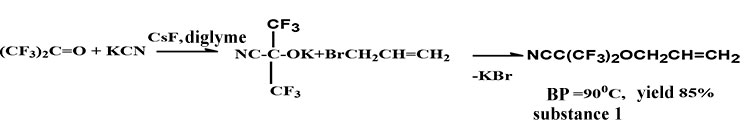
Scheme 1
In the process of hydrosilylation of obtained allyl ether by dimethylchlorosilane in the presence of 0.1N solvent H2PlCl6 in THF, compared to well known method [4], C6F6 was applied as a solvent, which one highly dissolved not only starting compounds but also reaction products. As a result it was obtained correspondent monochlorosilane with 85% yield. It was synthesized fluorocontaining disiloxane by hydrolysis of this monochlorosilane. A reaction control was made by NMR spectrum. In spectra of reaction products with conversion increasing there were appeared signals in the areas, ppm: 3.80 (OCH2), 1.02 (SiCH2) and 0.76 (SiCH3). In addition there were disappeared signals in area, ppm: 5.30 (=CH2) and 5.87 (CH=). An yield is 85%.
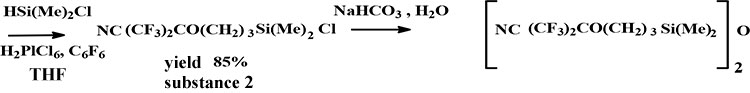
Scheme 2
In the process of hydrosilylation of above mentioned HFA cyanohydrin allyl ether by methyldichlorosilane in analogical conditions was obtained correspondent dichlorosilane. In the process of fluorocontaining dichlorosilane hydrolysis in the presence of sodium carbonate at temperature 00С there were obtained correspondent silanedioles. In the process of stabilization of fluorocontaining silanediols by trimethylchlorosilanes it was synthesized oligomer with molecular weight 1920 and glass transition −78°С (scheme 3):
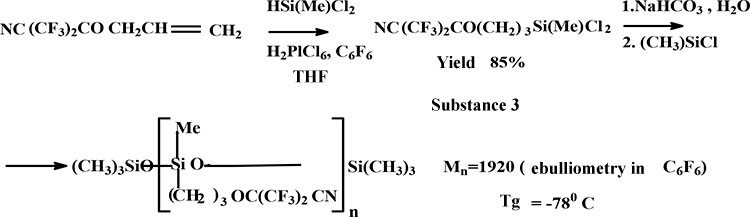
Scheme 3
Fluorocontaining silanedioles (scheme 4) were subject to catalytical polycondensation with hexamethylcyclo trisiloxane in the presence of sulphuric acid. For terminal silanol group stabilization of synthesized oligomers the last one were treated by trimethylchlorosilane:
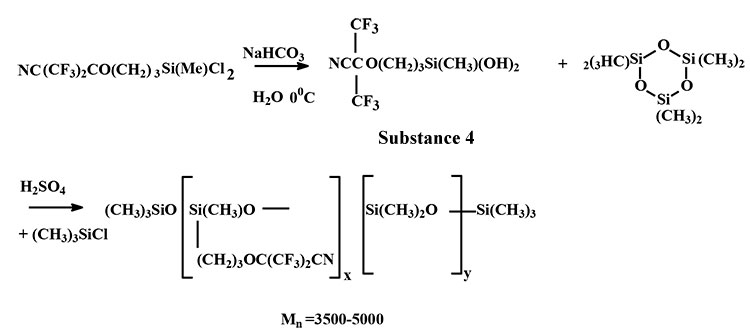
Scheme 4
There were obtained oligomers containing cyanoperfluoroalkyl classification in a side chain of macromol, it could be applied as compounds for greases agents. A temperature of initiation of decomposition was 350°С.
It is known that introduction of aromatic or heteroaromatic cycles into chain of macromol often leads to significant increasing of thermal stability of polymers. In particular, a result deserving attention from a practical perspective is achieved at introduction of triazine cycles into a chain of macromol that are connected by perfluoroalkylene fragments [1]. Therefore there is some future of interest in a development of methods of synthesis of fluoroorganosilicon compounds containing triazine cycles. It was considered one of the method for production of polyfluoroorganosiloxanes containing triazine cycles in this article. A baseline information for the synthesis of such oligomers and polymers were obtained as follow:
In the process of interaction of diamidines of perfluorocarbonic acid with HFA cyanohydrin allyl ether is initially formed imidoilamidine intermediates, which one transferred into correspondent fluorocontaining triazine intermediates with 90% yield in the result of acylation - cyclodehydration reaction with fluoroanhydrides of perfluorocarbonic acids. The further reactions of their silylation by dimethylchlorosilane in the presence of H2PlCl6 leaded to the formation of polyfluoroorgano- α,ω-dichlorosilane. As a result of hydrolysis there were obtained oligomers with molecular weight 3500-5600 (ebullioscopy in C6F6). Synthesized oligomers were stabilized by their treatment by trimethylchlorosilane on last stage (scheme 5).
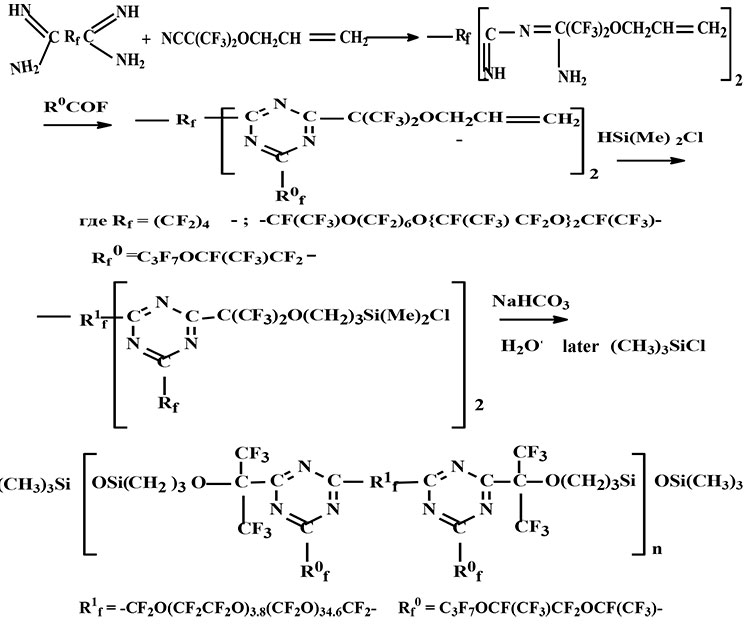
Scheme 5
These oligomers had a glass transition -70°С, 5% weight loss at 3600С and had high tribochemical properties. A friction coefficient (it is determined in All- Russia Research Institute of oil Refining) on four-ball machine in pair steel-bronze achieved 0.065. It was also found out the phenomena of selective transfer (it was occurred molecular film of bronze on steel).
Experimental
Spectra of NMR -19F and 1Н were registered on a spectrometer “Bruker WN-250» with a frequency 235 (reference standard CFCl3) and 250 MHz (reference standard ТМS), accordingly. Spectra 29Si were changed on a spectrometer «Bruker АС-200P» with a frequency 39,76 MHz (reference standard ТМS). IR-spectra were registered on a spectrometer «Specord IR-75». GCH-analysis was carried out on chromatography LHM-8MD (column 1000×3.5 mm, carrier Chromatone 0.160-0.200 mm, treated by 5% Silicon SE-30). starting chlorosilanes were bought from the company Acros Organics (the purity no less than 96%). A catalyst was prepared under the following procedure: 1 g of H2PlCl6 × Н2О were loaded into the flask containing 100 ml THF pre-distilled under СаН2, obtained solution was kept in closed flask at room temperature within 168 hours.
1. A synthesis of HFA cyanohydrin allyl ether (scheme 1):
A four neck 1 liter flask equipped with magnetic stirring, thermometer, sulfuric bottle and tube for HFA feeding was bubbled by nitrogen within 30 minutes. Into flask was loaded 56g (0.85 mole) of dry KCN, added 350 ml of anhydrous diglyme and 115g (0.65 mole) of HFA was feeding with 95g/hour rate under intensive stirring. A reaction temperature (by heat rise process) was kept at 33 0C at this speed of feeding. Than a reaction temperature was reduced to room temperature and 83.3g (0.39 mole) of allyl bromide was loaded through dropping funnel. The content of flask was heated to 80°С and stirred within 10 hours. A reaction mixture was cooled, residue was filtered (potassium bromide and unreacted potassium cyanide). According to GCH data the mixture consists of diglime and HFA cyanohydrin allyl ether only. It was obtained 136 g (85%) of target product after rectification with b.p. .= 90°С, d420=1.2660, nD20 =1.3263. NMR spectrum -1Н (DMSO d6), δ, ppm: 3.8 (d. 2Н, ОСН2); 5.30 (m.1 Н, CH=) and 5.87 (d. 2Н, CH2=). NMR spectrum-19F, (CFCl3), δ, ppm: -81.7 (s. 6F, СF3). Elemental analysis, found, %: C 36.00; 36,29; H 2.20; 2.23; N 5.85; 5.90; F 51.00; 50.60. Calculated for C7H5NF6O, %: C 36.10; H 2.16; N 6.00; F 49.80.
2. Hydrosylilation of compound 1 (scheme 2):
Three neck flask equipped with magnetic stirrer, dropping funnel and reflux condenser was loaded 23.3 g (0.1 mole) of HFA cyanohydrin allyl ether, 20 ml of C6F6, 10 g (0.1 mole) of (СH3)2SiHCl and 0,07 g of 0.1N solution H2PlCl6 in THF. A flask was heated up 60°С within 10 hours. A reaction mixture was distilled and fraction with b.p.=70°/30 torr separated. An yield was 23.5 g (85%). NMR spectrum -1Н (DMSO d6), δ, ppm: 0.76 (s. 6H, Si-CH3); 1.02 (t. 2Н, Si-CH2); 1.8 (m. 2 Н, CH2 CH2 CH2); 3.8 (t. 2Н, ОСН2); 4.48 (t. 2Н, CH2). In spectrum there are no signals 3.6; 5.30 and 5.87, typical for group ОСН2CH=CH2 (accordingly). NMR spectrum -19F, (CFCl3), δ, ppm: -81.7 (s. 6F, СF3).
3. Synthesis of fluorocontaining oligosiloxane (scheme 3):
Synthesis of fluorocontaining dichlorosilane based on compound 1 was carried out according to clause 2, but methyldichlorosilane was used as sylilating agent. Fraction with b.p.= 90°/20 torr was separated. NMR spectra of compound 3 are analogical to the above mentioned. 10.4 g (3×10-2mole) of compound 3 was loaded into the flask cooled by ice, equipped with stirrer and dropping funnel. Then it was added 10 ml of benzene and 20 ml of 5% aqueous solution of Na2CO3 was added at intensive stirring. Aqueous layer was separated, Na2SO4 was dried. It was obtained correspondent fluorocontaining silanediol (compound 4, scheme 4) as an intermediate. benzene solution of compound 4 was boiled with reflux condenser within 4 hours, cooled and add 1 ml of (CH3)3SiCl. It was obtained oligomer with Мn=1920, glass transition = -78°С.
4. Catalyst polycondensation of compound 4 and hexamethylcyclo-trisiloxane (HMCTS) (scheme 4):
Into flask equipped with stirrer, thermometer, dropping funnel and reflux condenser was loaded 10g (3.2×10-2mole) of compound 4 and 13.3 g (6.4×10-2mole) of HMCTS and three drops of concentrated H2SO4. A mixture was intensively stirred and heated to 90°С within 5 hours. Then 10 ml of benzene and 1 ml of (CH3)3SiCl were added. It was stirred within 1 hour and washed by water up to neutral reaction. Benzene solution was separated through separation funnel and dried Na2SO4. Benzene was separated through vacuum and it was obtained 20 g if viscous oligomer with Мn =5000 (ebullioscopy in C6F6).
5. Synthesis of perfluorotriazine oligomers with terminal allyloxy- and chlorosylil groups (scheme 5):
5.1.
Into vacuum ampoule was loaded 30.3g (1.1×10-2 mole) of NCCF2O(CF2CF2O)3.8(CF2O)34.6CF2CN with molecular weight 2720 (ebullioscopy in C6F6) and 5.6 g of liquefied ammonia (3.2×10-1 mole). Sealed ampoule was kept within 24 hours at 20°С, the same as [5]. After separation of surplus of ammonia it was obtained oligoamidine with molecular weight 6870 (ebullioscopy in C6F6). Obtained product was dissolved in 50 ml of C6F6) and 7.75g (3.3×10-2mole) of compound 1 was added. A mixture was stirred within 20 hours, then 32 g (7×10-2 mole) of fluoroanhydride dimer oxide of hexafluoropropylene [6] was added. Volatile products are distilled under vacuum at 100°С/10 mm. It was obtained 28g of oligomer with Мn=7140 (ebullioscopy in C6F6). IR spectrum, ν, cm-1: 1000-1200 (C-F bound); 1560 (C=N-triazine cycle); 1680 (CH=CH2). NMR spectrum -1Н, (DMSO d6), δ, ppm: 4.48 (d.2H, О-СН2); 5.30 (d.2H, СН2=) and 5.87 (m. 1H, СН=). NMR spectrum-19F (CFCl3), δ, ppm: two signals in the area -53.5 (CF2O)34.6 ; -70.3 [s. 6 F, C(СF3)2]; -76.5 (2F OCF2-of triazine cycle; a group of signals in the area -82.6 and -84.48( CF3 and CF2O) in group СF3CF2CF2O; two signals in the area -87.7 and -84.48 4 F in the group (CF2CF2O)3.8; -132.2 CF2; in the group СF3CF2CF2O; -147 CF(CF3)CF2O.
Elemental analysis: found, %: C 19.53; 19.39; H 0.05; N 1.35; 144; F 56.13; 55.51. Calculated: C122H10N13F216O89. C 20.5; H 012; N 2.4; F 57.07 O 19.86
5.2. Bis-perfluoroalkyltriazine with terminal chlorosilyl groups
Into flask with magnetic stir 2.84 g (4×10-4 mole) of obtained above oligofluorotriazine was loaded, 10 ml C6F6, 0.23g (2.4×10-3mole) of dimethylchlorosilane and 0.066 g of 0.1N solvent H2PlCl6 in THF. A flask was sealed up and heated on oil bath at stirring at 70°С within 20 hours. A flask was opened, content was filtered, volatile products were deleted under vacuum. It was obtained 2.74g (89.5%) of oligomer with terminal chlorosilyl groups. Мn=7500 (ebullioscopy in C6F6). glass transition= -72°С. IR spectrum, ν, sm-1: 1000-1200 (C-F bound); 1410 (СН- bound in Si-СH3); 1560 (C=N-triazine cycle); 1680 (CH=CH2), 2800-3000(С-Н- bound of СН2 groups).
NMR spectrum-1Н (DMSO d6), δ, ppm: 0.76 (s. 3Н, Si-CH3); 1.02 (t. 2Н, Si-CH2); 1.8 (m. 2Н, CH2 CH2 CH2); 3.8 (t. 2Н, ОСН2); In spectrum there are no signals at 4.48; 5.30 and 5.87 ppm, typical for ОСН2CH=CH2 classification. Accordingly, reaction of addition was on going on practically quantitatively.
NMR spectrum -19F, (CFCl3), δ, m d: two signals in the area -53.5 (CF2O)34.6 ; -70.3 [s. 6 F, C(СF3)2]; -76.5 (2F OCF2-of triazine cycle; signal group in the area -82.6 and -84.48( CF3 and CF2O) in the group СF3CF2CF2O; two signals in the area -87.7 and -84.48 4 F in the group (CF2CF2O)3.8; -132.2 CF2; in the group СF3CF2CF2O; -147 CF(CF3)CF2O.
Elemental analysis: found, %: C 22.24; 22.00; H 0.22; 0.25; N 2.75; 2.86; F 60.48; 60.95; Cl 1.21 1.17. Calculated for C187H18N19F235Si12Cl4O67 , %: C 22.44; H 0.18; N 2.73; F 61.84; Si 0.56; Cl 1.42; O 10.64.
References
- V.A. Ponomarenko, S.P. Krukovskij, A.Yu. Alybina. Ftorsoderzhashchie geterocepnye polimery. M. Nauka, s.257, 1973g.
- V.A. Ponomarenko, M.A. Ignatenko. Khimiya ftorkremnijorganicheskih soedinenij. M., Nauka, s. 191, 1979g.
- A.A. Yarosh, A.A. Glazkov, A.M. Saharov. Tezisy doklada H111 Andrianovskoj konferencii «Kremnijorganicheskie soedineniya. Sintez, svojstva, primenenie». Moskva, 2015g, RO 90, str. 168.
- S.P. Krukovsky, A.A. Yarosh, A.A. Glazkov, D.V. Batizat, T.N. Redina. J. Fluorine Chemistry, 96 (1999), p. 31-33.
- Reily W.L., Brown H.G. J. Org. Chem. 1957, V.22, p.698
- I.L. Knunyants, A.V. Fokin, Yu.A. Cheburkov. ZhVKhO im. D.I. Mendeleeva, 13, N 3, 311, (1968).
Recommended for publication by Prof. S. P. Krukovsky
Fluorine Notes, 2016, 104, 1-2
