Received: July, 2015
DOI 10.17677/fn20714807.2015.06.03
Fluorine Notes, 2015, 103, 7-8
Polyfluoroalkyl –tert-butylperoxicarbonates as effective initiators for fluoropolymers structurization
N.A.Rakhimova1, L.A. Butkovskaya1, A.I. Rakhimov1,2
1Volgograd State Technical University, 400131, Russia, Volgograd, Lenin prospect,
28
e-mail: organic@vstu.ru
2Institute of Chemical problems of Ecology ANS RF, 400066, Volgograd PO box 127,
e-mail: rakhimov@sprint-v.com.ru
Abstract: The effect of polyfluooroalkyl –tert butylperoxicarbonates as structural agents on process of vulcanization of fluorocaoutchoucs SKF-32 and SKF-26 and on improvement of properties, obtaining by vulcanization is shown.
Keywords: Polyfluooroalkyl –tert butylperoxicarbonates, initiators for fluoropolymer structurization, structure films SKF-32 and SKF-26
Introduction
The development of methods doping of polyfluoro- groups into macromolecular systems is an actual task, since it lets to improve considerably physical and mechanical properties of polymer composite materials. [1-11]
Fluorocaoutchoucs have special position in technology, and are characterized with high resistance to strong oxidizing agents and aggressive environment, nonflammability, thermal resistance and necessary stability characteristics. [12,13]
The aim of this work is to consider a possibility of vulcanization of fluorocaoutchoucs with polyfluoro –tert-butylperoxialkyl carbonates. Fluorocaoutchoucs SKF-32 ans SKF-26, which are commercially produced, contain large quantity of CH2 groups, and though indistinct liability of CH2 groups in f fluorocaoutchoucs to homolytical reactions, the complex of these groups and double bonds (which are formed in the structure of polymer by high temperatures in the presence of halogen hydrides acceptors) provides fluorocaoutchoucs an ability to vulcanize with peroxides. [13]
Discussion of results
It is necessary mention, that peroxides, which are used by structurization of fluoropolymers, such as benzoyl -, cumyl -, tert–butyl peroxide, have some technical disadvantages: cumyl peroxide forms highly toxic acetophenone by decomposition; benzoyl peroxide is explosive, di- tert.- butyl peroxide is volatile.
Esters of percarbonic acid have one of the most important place among highly effective initiators of peroxide type.
Polyfluoro–tert Butylperoxialkyl carbonates (PFTBPK) show the highest termal resistance [4-11].
Fluorinated peroxialkylcarbonates are obtained on the basis of polyfluoroalkyl esters of chlorocarbonic acid (PFECK) [7].
The synthesis of PFTBPK is carried out by interaction of corresponding chlorformate with mixture of tert-butylhydroperoxide and aqueous solution of sodium hydroxide in the presence of organic solvent by temperature 0° C. After blending of reagents, the temperature rises to room temperature and reaction ends in 2 hours.
Х(СF2)n СН2 ОС(О)Сl + (СН3 )3 СООН → Х(СF2)nСН2ОС(О)ООС(СН3)3
Where X= H or F, n = 1,2,4,6,8
Physico-chemical properties of polyfluorinated tert.-butylperoxialkylcarbonates are presented in the table 1.
Table 1. Physico-chemical properties polyfluorinated tert.-butylperoxialkylcarbonates Х(CF2)nCH2ОС(О)ООС(СН3)3
|
# |
Compound |
Yield % |
B point , °С. (р=133Па) |
nD20 |
d420 |
Gross -formula |
Composition of elements |
||||||
|
Х |
n |
Found,% |
Calculated,% |
||||||||||
|
C |
F |
Oакт |
C |
F |
Oакт |
||||||||
|
1 |
F |
1 |
73.7 |
31 |
1.3772 |
1.2159 |
C7H11O4F3 |
38.81 |
26.3 |
7.32 |
38.88 |
26.38 |
7.41 |
|
2 |
H |
2 |
80.0 |
46 |
1.3737 |
1.2678 |
C8H12O4F4 |
38.67 |
30.6 |
6.4 |
38.7 |
30.69 |
6.45 |
|
3 |
H |
4 |
75.4 |
55-56 |
1.3600 |
1.4061 |
C10H12O4F8 |
34.31 |
43.57 |
4.56 |
34.48 |
43.67 |
4.6 |
|
4 |
F |
6 |
70.8 |
73-75 |
1.3512 |
1.4946 |
C12H12O4F12 |
32.02 |
50.71 |
3.54 |
32.14 |
50.89 |
3.57 |
|
5 |
H |
6 |
74.5 |
51 |
1.3488 |
1.5401 |
C12H11O4F13 |
30.79 |
52.87 |
3.40 |
30.90 |
53.00 |
3.43 |
|
6 |
H |
8 |
65.5 |
113 |
1.3460 |
1.5801 |
C14H12O4F16 |
30.63 |
55.40 |
2.89 |
30.65 |
55.47 |
2.92 |
These are liquid substances with high density, which increases with growth of perfluorocarbon
chain to d420 = 1,5801 g/cm3 for tert.-butylperoxi 1,1,9-trihydroperfluorononanilcarbonate
, H(CF2)8CH2OC(O)OOC(CH3)3.
The obtained polyfluoro- tert-butylperoxialkylcarbinates were used as structuring agents of fluorocaoutchoucs (SKF-32 and SKF-26)
For this aim a resin mixture with equal molar content of peroxide was prepared. (PFTBPK, unlike benzoyl peroxide, is good mixable with fluorocaoutchoucs, thereby resin mixtures are not prevulcanised) . Peroxide was introduced by the end of component’s blending and its full allocation in resin mixture was observed. (5-10 minutes)
The structurization of samples was conducted in hydraulic press with electric heating and automatic temperature control of ranges under pressure of 10 MPa. Thereby, the speed of temperature increase was optimal (10 minutes by 180 ° for SKF-32 and 30 minutes by 160°C for SKF-26) After vulcanization the temperature of sample under pressure was gradually decreased to 60 °C (to avoid pore-formation in vulcanizate). The heat aging of obtained vulcanizates was conducted during 72 hours by 150°C and 24 hours by 200 °C.
The results are presented in tables 2 and 3. On the basis of obtained data, we can make a conclusion that structuring ability of used peroxides depends on structure, and therefore on their thermal resistance. The introduction of fluorine atom results in decreasing speed of thermal decay of peroxide and decreasing speed of structurization, which increase the density of vulcanization network. Moreover, the high structuring activity of PFTBPK is caused by formation of more active in the hydrogen-atom abstraction reaction polyfluoroalkoxil radicals.
Preparation of films from fluorocaoutchoucs by milling was conducted on laboratory rolling mills with intensive cooling of rolls. The temperature of rolls while mixing was held between 20 -25 °C. Peroxide was introduced to the end of mixing until its full allocation in resin mixture (5- 10 minutes), The vulcanization of samples was conducted after keeping for 6 hours by room temperature in hydraulic press with electric heating and automatic temperature control of ranges under pressure of 10 MPa. The speed of increasing temperature was not more then 2 %/ minute. After vulcanization the temperature of sample was gradually decreased to 60 °C , which is necessary to avoid pore-forming in vulcanizate.
The thermal resistance of obtained films (table 2,3) was tested by method of thermal differential analysis and it was found out that usage of PFTBPK results in increasing of onset temperature of vulcanizates till 280 °C ( the onset temperature for unsubstituted films 225 °C).
Table 2. Physical and mechanical properties of structured films on basis of SKF-32
|
Peroxide |
Mode of structurization°С in Min. |
Module 300 %, MPa |
Tensile strength MPa |
Extension coefficient,% | Persistentextension, % | Hardness by TM-2 |
Rebound elasticity, % |
| Benzoyl peroxide | 180х10 | 7,7 | 16,8 | 440 |
12 |
69 |
8 |
|
Н(CF2)2CH2ОС(О)ООС(СН3)3 |
180 х 10 |
7,9 |
18,7 |
510 |
8 |
70 |
10 |
|
Н(CF2)4CH2ОС(О)ООС(СН3)3 |
180 х 10 |
8,0 |
17,5 |
430 |
12 |
68 |
8 |
After thermal aging
|
Benzoyl peroxide |
150 х 72 200 х 24 |
- - |
12,5 15,0 |
430 400 |
- - |
- |
- - |
|
Н(CF2)2CH2ОС(О)ООС(СН3)3 |
200 х 24 |
- |
15,6 |
405 |
- |
- |
- |
|
Н(CF2)4CH2ОС(О)ООС(СН3)3 |
150 х 72 |
- |
13,0 |
380 |
- |
- |
- |
Table 3. Physical-chemical properties of structuring films on basis of SKF-26
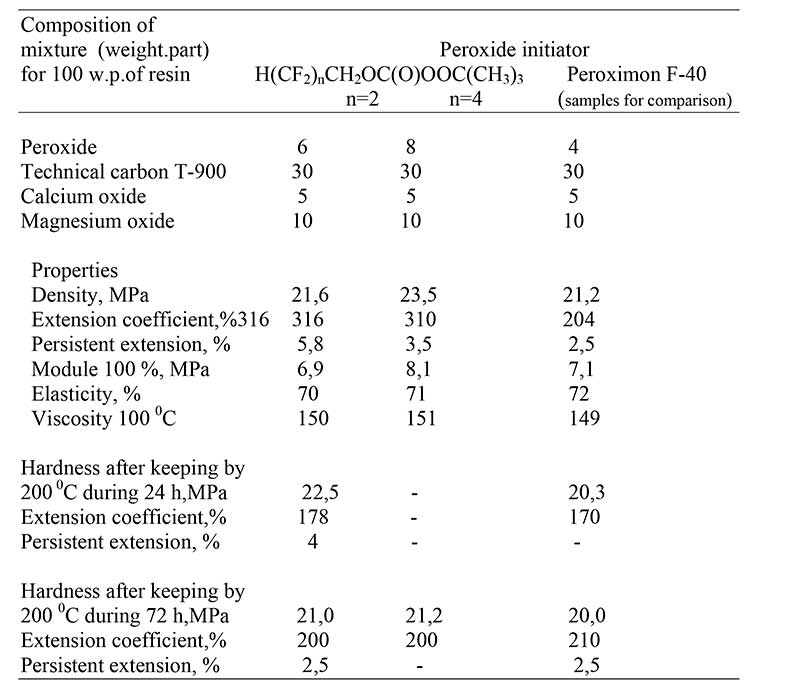
Peroximon
F-40 -ditret.-butylperoxide
Moreover, it is necessary to mention a considerable improvement in complex of physical and mechanical properties of obtained films for tert.-butylperoxitetrafluoropropylcarbonate: tensile strength for structuring films on the basis of SKF-32 is 18.7 MPa by high values of rebound elasticity (10 %).
A high efficiency of polyfluoroalkyl – tert –butyl peroxicarbonates as initiators for structurization of films for such fluoropolymers as SKF-32 and SKF-26 was determined.
References
- Rakhimov A.I. Methods doping of polyfluoro- groups into macromolecular systems. New perspective materials and technologies. Collection of scientific works 5- International conference. Volgograd. 2010, p.79-80.
- Rakhimov A.I. Chemistry and Technology of fluoroorganic compounds.M. Chemistry.1986. 271p.
- Rakhimov A.I., Krukova E.G., Bogath E.V. Sergeev S.A., Kostirja V.I. Pat.2021286. R.F. MPK S1F224.114/08.1994.
- Rakhimov A.I., Bogdanova O.S.,Butkovskaj L.A. Obtaining of suspension polyvinylchloride with help polyfluoroperoxydicarbonates. Chemistry and polymeric materials. Collection of scientific works of Volgograd State Technical University. Volgograd. 1999.p. 78-88.
- Rakhimov A.I. Chemistry and Technology of organic Peroxides. M. Chemistry.1979. 389 p.
- Rakhimov Alexander. Initiators for Manufacture of PVC. New York. Nova Science Publishers. 2008. 181 p.
- Rakhimov A.I., Butkovskaj L.A., Baklanov A.V. Synthesis of polyfluoro – tert. – butylperoxyalkylcarbonates. Chemistry and Technology element organic monomeric and polymeric materials. Volgograd .2008,v.39, №1, p.85.
- Rakhimov A.I., Butkovskaj L.A. Particularities of thermolysis for di(polyfluoroalkyl) peroxydicarbonates and theirs application.Fluorine Notes J. №3, (82), 2012.
- Rubanova R.A., Androsuk E.R., Rakhimov A.I. Investigation of activity for fluorobenzoyl peroxides in process of structured elastomers. Volgograd conference.1980, 300 p.
- Rakhimov A.I., Butkovskaj L.A. The influence of dimethylenoxide group in polyfluoroalkylperoxydicarbonates on destraction Process.. Fluorine Notes J,2013. №2, (87),
- Rakhimov A.I., Butkovskaj L.A. Synthesis and properties of di(polyfluoroalkyl peroxy)dicarbonates. J.General Chemistry 2011,v.81, №5, p.889.
- Galyl- Ogli F.A.,Novikov A.S., Nudelman Z.N. Fluorocaoutchoucs and rubbers on them base. M.Mir,1975, 240-260 p.
- Pasiorerek K. Structuretion of caotchoucs. In Fluoropolymers. M. Mir.1975.
Recommended for publication by Prof. A. Rahimov
Fluorine Notes, 2015, 103, 7-8
