Received: October, 2015
DOI 10.17677/fn20714807.2015.05.03
Fluorine Notes, 2015, 102, 5-6
Synthesis and Research of Optical properties of tert-perfluorinated esters of alpha-fluoroacrylic acid
V. E. Boykoabc, A.A. Tyutyunovabc, A.V. Sin’koabc, S.M. Igumnovabc, E. V. Khaidukovb, V. I. Sokolovb
aA.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, V-334, GSP-1, 119991 Moscow, Russia
bNPO PiM-INVEST LLC, ul. Vavilova 28, 119991 Moscow, Russia
cThe Institute on Laser and Information Technologies of the Russian Academy of Sciences, 1 Svyatoozerskaya St. 140700 Shatura, Moscow Region, RUSSIA
e-mail: boykii@mail.ru
Abstract: Group of tertiary perfuorinated ether alpha-fluoroacrylic acid type CH2=CF-CO2-C(CF3)2-(CF2)m-CF3 where m= 0, 1, 3, 5 containing degree of fluorination from 83.3 to 90.9% were prepared. Synthesized α-fluoroacrylates (1) have high transparency in all three telecommunication ranges 0.85, 1.3 and 1.5 μm. Refractive index of monomers decreases with elongation m of aliphatic perfluorinated radical and comes to 1.313 – 1.316.
Keywords: α-fluoroacrylates, coefficient of absorption, refractive index, integrated optics.
Polymers on basis of fluorinated acrylates find wide application in industry, science and technology. [1-17]. One of the most promising field of application is fiber and integrated optics,
where acrylates are used as materials for creation of optical fibers and waveguides, providing high speed interconnection in “telecommunication” wavelength ranges near λ = 0.85, 1.3 and 1.55 mcm [4-9, 18-21]. Thereby the highest optical transparency in above spectral ranges have acrylates with minimum content of C-H bonds, that means with maximum degree of fluorination [22-26].
To increase the degree of fluorination of acrylic monomers by keeping its high activity in the process of radical UV photopolymerization, we developed methods of synthesis for cyanoperfluoro- cyanochloroperfluoroalkylacrylates, and also tert-butyl-alpha-fluoroacrylates. [27,28]. The next step to increase the degree of fluorination of acrylic monomers is transfer to α-fluoroacrylates of perfluorinated tert-alcohols with extended length of aliphatic radical in alcohol part of an ester. For this purpose, we developed method of synthesis of new, previously not described monomers of homologues series
CH2=CF-CO2-C(CF3)2-(CF2)m-CF3, (1)
where m = 0, 1, 3, ,5, with degree of fluorination from 83.3 to 90.9 %. The synthesis of α- fluoroacrylates (1) was conducted according to the below Scheme 1 through interaction of obtaining in situ potassium salts of perfluorotertbutyl alcohols , reaction of nucleophilic addition of perfluoroalkyl(trimethyl)silanes to hexafluoroacetone by the presence of equimolar quantity of Potassium fluoride with 2,3-dichloro-2-fluoropropionyl chloride and following dechlorination.
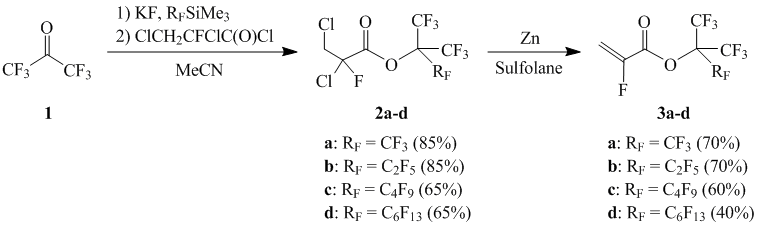
Scheme 1
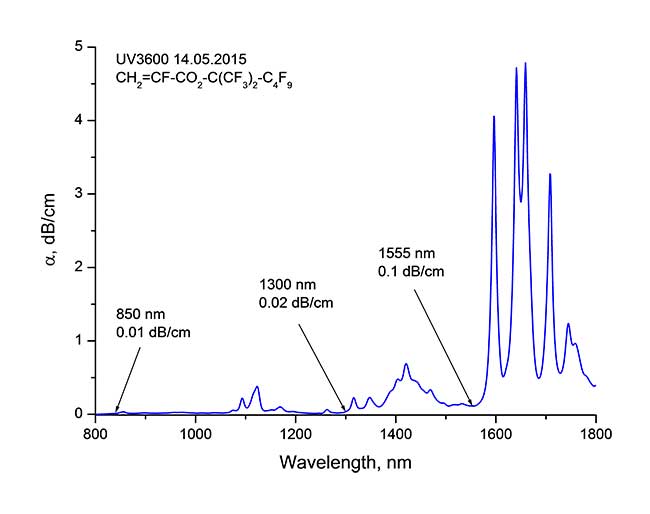
Investigation of optical properties of monomers showed, that synthesized α-fluoroacrylates (1) have high transparency in all three telecommunication ranges 0.85, 1.3, 1.5 mcm, which is provided by their high range degree of fluorination, reaching 90.9 % . Typical absorption spectrum of these monomers, provided in dB/cm is presented in picture 1. It can be seen, that attenuation coefficient comes to 0.01 dB/cm (λ = 850 nm), 0.02 dB/cm (λ =1300 nm) and 0.1 dB/cm (λ=1550 nm) correspondingly.
|
|
Fig. 1. Attenuation coefficient [dB/cm] α- fluoroacrylates CH2=CF-CO2-C(CF3)2-C4F9 in telecommunication ranges near 0.85, 1.3 и 1.5 μm. |
Refractive index nD on wavelength λ = 589.3 μm and average dispersion nF – nC of monomers (1) were measured with the help of Abbe refractometer IRF-454 B2M and are presented in the table 1. Table 1 shows, that refractive index of monomers decreases with elongation m of aliphatic perfluorinated radical (that means with increase of degree of fluorination) and comes to 1.313 – 1.316 , which is record-low for UV photocurable acrylates. Refractive indexes of homopolymers, produced from monomers (1), rather higher and fall within the range of 1.343 – 1.349. It is explained with weight loss and altering of chemical structure in the process of radical polymerization (opening of double C=C bonds, building of polymer’s macromolecules from monomer molecules and ets.)
Table. 1. Refractive index nD on wavelength λ = 589.3 μm and average dispersion nF – nC of fluorocontaining α-fluoroacrylating monomers (1), take under 20°C.
|
Chemical Formula |
Conversion of fluorination, % |
nD |
nF - nF |
|
CH2=CF-COO-C(CF3)3 |
83.3 |
1.3160 ± 0.0002 |
6.521 × 10-3 |
|
CH2=CF-COO-C(CF3)2-(CF2)-CF3 |
85.7 |
1.3156 ± 0.0002 |
6.520 × 10-3 |
|
CH2=CF-COO-C(CF3)2-(CF2)3-CF3 |
88.9 |
1.3148 ± 0.0002 |
6.516 × 10-3 |
|
CH2=CF-COO-C(CF3)2-(CF2)5-CF3 |
90.9 |
1.3137 ± 0.0002 |
6.512 × 10-3 |
The synthesized α-fluoroacrylic monomers (1) have high activity in the process of radical UV photopolymerization, which can be initiated without adding any photoinitiators under the action of UV radiation with wavelength shorter then 260 nm. These monomers are suitable for production of optical waveguides by methods of direct laser writing or UV photolithography.
Experimental
NMR 1H, 19F spectra were recorded on spectrometer “Bruker AVANGE-300” by 300 and 282 MHz correspondingly, external standard is CDCL3. Chemical shifts for 1H spectra are provided in m.d with respect to tetramethylsilane. Chemical shifts of 19F specra are provided in m.d with respect to CFCL3. The purity of monomers were measured by method of gas-liquid chromatography (GC) on chromatograph Shimadzu 2010 + column Resteck RTX-1701 (USA) 14 % cyanopropylphenyl, 86 % polysiloxane, length 30 m, 0,32 ID, 1 m df, flame ionization detector, carrier gas nitrogen.
General method of synthesis of fluoroacrylic monomers
To a suspension of potassium fluoride (30 g, 0.516 mol) in acetonitrile (200 ml) by stirring and temperature -30 - -25 oC gaseous hexafluoroacetone is added (85 g, 0,512 mol) and RFSiMe3 (0,512 mol). After completion of exothermic reaction ,as a result of which the temperature of reaction mixture rises to 0 – 5 °C , the mixture is stirred by 15 – 20 °C during 2-3 hours, cooled to 0 -5 °C and ClCH2CFClC(O)Cl ( 92 g, 0,513 mol) drop-wise is added. The mixture is stirred by 15 -20 °C during 2-3 hours, poured in water , the bottom layer is separated , washed 4 times with water, P2O5 (15g) is added and rerun in vacuum ( 10 -0,5 torr). The product is purified by redistillation.
1,1,1,3,3,3-Hexafluoro-2-(trifluoromethyl)propan-2-yl-2,3-dichloro-2-fluoropropanoate (2a)
bp 50 -52 °C/15 torr
NMR 1H δ: 4,0÷4,2 (m, CH2Cl); NMR 19F δ: -125,4 (m, 1F, CFCl), -71,7 (m, 9F, CF3).
1,1,1,3,3,4,4,4-Octafluoro-2-(trifluoromethyl)but-2-yl-2,3-dichloro-2-fluoropropanoate (2b)
bp 73-75оС/15 torr
NMR 1H δ: 3,9÷4,2 (m, CH2Cl); NMR 19F δ: -125,4 (m, 1F, CFCl), -118,5 (m, 2F, CF2), -81,8 (m, 3F, CF3), -69,0, -68,6 (m, 6F, CF3).
1,1,1,3,3,4,4,5,5,6,6,6-dodecafluoro-2-(trifluoromethyl)hex-2-yl-2,3-dichloro-2-fluoropropanoate ( 2c)
bp. 110-113оС/15 torr
NMR 1H δ: 3,9÷4,1 (m, CH2Cl); NMR 19F δ: -127,8 (m, 2F, CF2), -125,3 (m, 1F, CFCl), -122,2 (c, 2F, CF2), -113,8 (m, 2F, CF2), -83,5 (m, 3F, CF3), -68,6, -68,2 (m, 6F, CF3).
1,1,1,3,3,4,4,5,5,6,6,7,7,8,8,8-hexadecafluoro-2-(trifluoromethyl)octan-2-yl-2,3-dichloro-2-fluoropropanoate (2d)
bp 100-103оС/0,5 torr
NMR 1H δ: 4,0÷4,2 (m, CH2Cl); NMR 19F δ: -127,3 (m, 2F, CF2), -125,4 (m, 1F, CFCl), -122,2 (m, 4F, CF2), -121,5 (m, 2F, CF2), -113,8 (m, 2F, CF2), -83,6 (m, 3F, CF3), -68,6, -68,2 (m, 6F, CF3).
To a suspension of zinc powder ( 9,81 g 0,15 g-atom) activated by adding of trimethylchlorosilane (1ml) in sulfolane (50ml) in vacuum 10 – 0,5 torr by stirring and temperature 30 -40 °C ester 2a-d drop-wise is added (0,1 mol), the reaction product is distilled into cooled till 0°C receiver. The obtained product is additionally purified by rectification. The purity of monomers by GC comes to 96-99 %.
1,1,1,3,3,3-Hexafluoro-2-(trifluoromethyl)propan-2-yl-2-fluoroacrylate (3a)
bp 19-20оС/15 torr
NMR 1H δ: 5,7÷6,2 (m, CH2=); NMR 19F δ: -119,4 (m, 1F, CF), -71,7 (m, 9F, CF3).
1,1,1,3,3,4,4,4-Octafluoro-2-(trifluoromethyl)but-2-yl-2-fluoroacrylate (3b)
bp 28-30оС/15 torr
NMR 1H δ: 5,7÷6,2 (m, CH2=); NMR 19F δ: -119,4 (m, 1F, CF), -118,5 (m, 2F, CF2), -81,8 (m, 3F, CF3), -69,0, -68,6 (m, 6F, CF3).
1,1,1,3,3,4,4,5,5,6,6,6-Dodecafluoro-2-(trifluoromethyl)hex-2-yl-2-fluoroacrylate (3c)
bp 70-71оС/15 torr
NMR 1H δ: 5,7÷6,2 (m, CH2=); NMR 19F δ: -127,8 (m, 2F, CF2), -122,2 (c, 2F, CF2), -119,4 (m, 1F, CF), -113,8 (m, 2F, CF2), -83,5 (m, 3F, CF3), -68,6, -68,2 (m, 6F, CF3).
1,1,1,3,3,4,4,5,5,6,6,7,7,8,8,8-Hexadecafluoro-2-(trifluoromethyl)octane-2-yl-2-fluoroacrylate (3d)
Bp 60-63оС/0,5 torr
NMR 1H δ: 5,7÷6,2 (m, CH2=); NMR 19F δ: -127,3 (m, 2F, CF2), -122,2 (m, 4F, CF2), -121,5 (m, 2F, CF2), -119,4 (m, 1F, CF), -113,5 (m, 2F, CF2), -83,6 (m, 3F, CF3), -68,9, -68,4 (m, 6F, CF3).
Work has been executed due to the financial support of RSCF (Russian Scientific Foundation) grant 14-19-01659.
References
- Akira O., Takahiro K. Application 60-78941 Japan// RJChem. 1986, 78Н67.
- Akira O., Takahiro K. Application 60-78942 Japan// RJChem. 1987, 7Н68.
- Akira O., Takahiro K. Application 60-78943 Japan// RJChem 1986, 7Н69.
- Bosc Ό., Boutevin В., Pietrasanta Υ., Rousseau А. Application 2623510 France// RJChem. 1990, 4С588.
- Jjaa S., Koji N., Maseru M., Takashi I. Application 61-121005 Japan // S. А. 1986.V. 105, 192481.
- Takashi I., Katsuhiko S., Ryuji M. et al. Application 61-240205 Japan // S. А. 1987.V. 106, 139496.
- Tategami Υ., Fujiia К., Furuta Μ., Tamura Т. Application 60-250309 Japan // S. А. 1986.V. 104, 226030.
- Joshiharu Т., Katsuramaru Т., Motonobu F., Tashibubnu Т. Application 61-208006 Japan // S. А. 1987. V. 106, 68407.
- Shigeru Μ., Masahiko О. Application 61-86448 Japan // S. А. 1986. V. 105, 157727.
- Akira O., Nobuyuki T. Takahiro K. Application 58-196218 Japan// RJChem. 1985, 8Ф50.
- Akira О., Kazuo I. Пат. 158113 Europe// S. А. 1985. V. 104, 89225.
- Akira О., Tahashi Y., Naoaki I., Yasyfymi U. Pat. 180913 Europe// S. А. 1986.V. 105, 154307.
- Akira O. Ya Naos, Kaoteru I. Kharusi U. Application 62-127306 Japan// RJChem.1988, 17Т122.
- Akira O., Naofumi Ya Naonki I. Yasusya U.. Application 61-111309 Japan// RJChem.1987, 100630.
- Akira O. Nobuyuki T. Application 61-141711 Japan// RJChem. 1987, 12Т402.
- Akira O. Nobuyuki T. Application 61-186924 Japan// RJChem. 1987, 20Т366.
- Sho Ya, Shigeaki K., Akira O., Kazuo I. Application 62-33110 Japan// RJChem. 1988
- Boguslavskaia L.S., Samarin A.V., Lebedev V.I. ets. CPlastics. 1988 .# 12. p. 15.
- Akira О., Nobuyuki Т., Sakahiro К. Pat. 128516 Europe// S. А. 1985. V. 102.185964 h
- Akira О., Nobuyuki Т., Takahiro К. Pat. 128517. Europe// S. А. 1985. V. 102115070 g.
- Akira О., Takahiro К., Takahiro T., - Application 60-118808 Japan// RJChem. 1987 4161.
- Rouge D., Gault H.I/Compt. rend. 1960. V. 251. P. 95.
- Sedlak J. Pat.. 3075002 USA// S. A. 1963. V. 59. 5027 h.
- Bergmann E. D., Shahak .// J. Chem. Soc. 1960. P. 5261.
- Ueda M., Yasawa m..// Fluorine chem. and its application. 1985. № 10. P. 1862.
- Tolman V., Spronglova P.// Ibid. 1983. V. 48. P. 319.
- Boyko V.E., Molchanova S.I., Sinko A.V., Sokolov V.I., Tyutyunov A.A., Khaidukov E.V., Igumnov S.M. // Fluorine notes, Number 6(97) 2014
- Tyutyunov A.A., Boyko V.E., Sinko A.V., Igumnov S.M., Molchanova S.I., Khaidukov E.V., Sokolov V.I.. // Fluorine notes, 6(97) 2014
Recommended for publication by Prof. S. M. Igumnov
Fluorine Notes, 2015, 102, 5-6