Received: April, 2015
DOI 10.17677/fn20714807.2015.04.01
Fluorine Notes, 2015, 101, 1-2
SURFACE MODIFICATION OF ISOTACTICAL POLYPROPYLENE BY POLYFLUOROALKYL GROUPS
A.I. Rahimov1, N.A.Rahimova2, V.S. Avilova2, E.V. Petrosyan 2, A.J. Marishev2., V. F. Zheltobryuhov 2
1Institute of Chemical problems of Ecology ANS RF, 400066, Volgograd PO box 127,
e-mail:
rakhimov@sprint-v.com.ru
2Volgograd State Technical University, 400131, Russia, Volgograd, Lenin prospect, 28
e-mail:
organic@vstu.ru
Abstract: Here you can see the opportunity of introduction of polyfluoroalkyl groups by oxidizing functionalization of polypropylene using air oxygen with further octafluoropentylchlorosulphite modification by HO-groups on the surface of fine-dispersed particles of isotactic polypropylene, what leads to obtaining of polyfluoroalkylated polypropylene with the complex of unique properties.
Keywords: Isotactical polypropylene, air oxygen, oxidizing functionalization of HO-groups, octafluoropentylchlorosulphite, polyfluoroalkylated surface, hydrophobic behavior, homogeneous spherical structure of particles.
The aim of the present work is the introduction of HO-groups into the surface of fine-dispersed particles of isotactic polypropylene (IPP) by its oxidizing functionalization with air oxygen and further polyfluoroalkylation of HO-groups by octafluoropentylchlorosulphite to obtain polyfluoroalkylated polypropylene. Oxidized IPP (OIPP) as stated by authors [9-17] is highly reactive. It reacts with polyisocianates and can be applied as modifier of composition materials.
1. Oxidation of Isotactic Polypropylene (IPP) By Air Oxygen
Oxidation of IPP was carried out in o- xylene (mass ratio 1:4) over 0,5 % wt. of cumene (isopropylbenzene) at 140° C for 3.5 hours. The oxidation process was observed by the concentration of HO-groups in oxidized polypropylene (OIPP), which had been determined by acylation of polymer using phthalic anhydride in pyridine. The concentration of OIPP has been analyzed every 30 minutes for 3.5 hours by the amount of forming acyl derivative (Pic. 1).
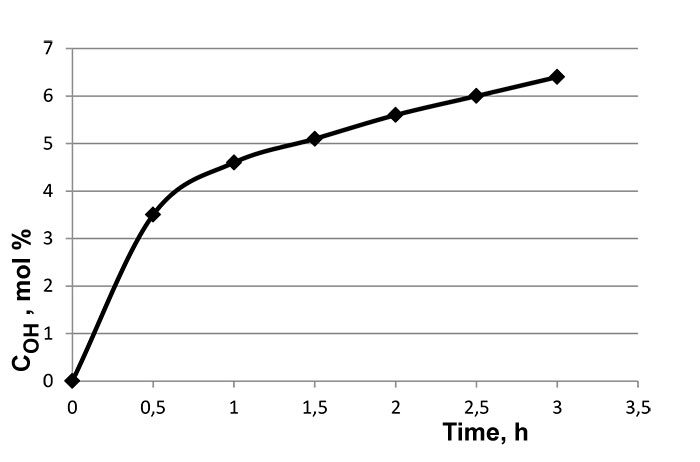
Pic.1. Alteration of Concentration of HO-groups in OIPP in the Process of Oxidation of IPP (Oxidation is carried out in o-xylene in the presence of 0,5 % wt. of isopropylbenzene) at 140° C.
Maximum concentration of OIPP in the products of oxidation is about 6%. The reaction of oxidation of IPP is accompanied by destruction of IPP micro-molecular chains, what leads to decreasing of viscosity of OIPP xylene solution. The structure of IPP oxidation products was studied using method FTIR spectroscopy. In the IR-spectrum (Pic. 2, 3) of oxidized polymer we can point out following characteristic stripes of absorption: 800-1000 cm-1, that corresponds to the oscillations of C-C-bond, as well as frequencies in the fields of 1380, 1450-1470 cm-1 referred to deformation oscillations of СН2- and СН3- groups and symmetrical oscillations of СН2- at frequencies of 2840-2860 cm-1 and СН3- group at 2860-2880 cm-1; as well as assymetric oscillations at 2920-2935 cm-1 and 2950-2970 cm-1, that corresponds to СН2- and СН3- groups. Besides that, in IR-spectra of OIPP there are absorption bands of carbonyl group at frequencies of 1700-1720 cm-1, and Нo-group in the field of 3400-4000 cm-1.
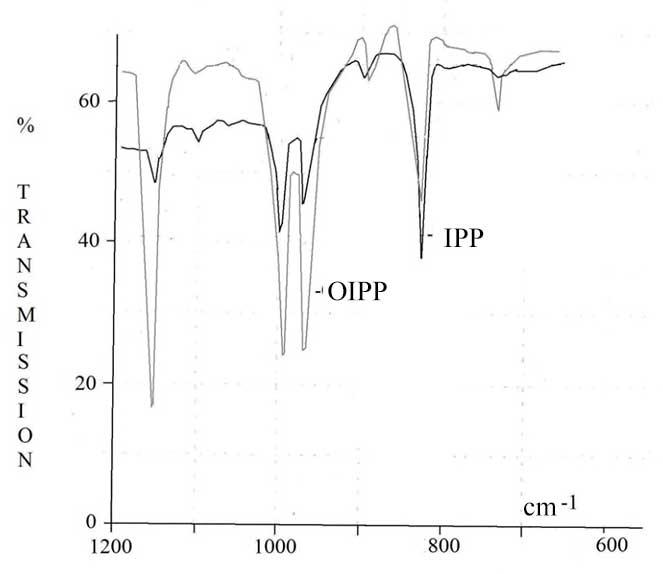
Pic. 2. FTIR Spectra of IPP and OIPP in the Field of 600-1200 cm-1
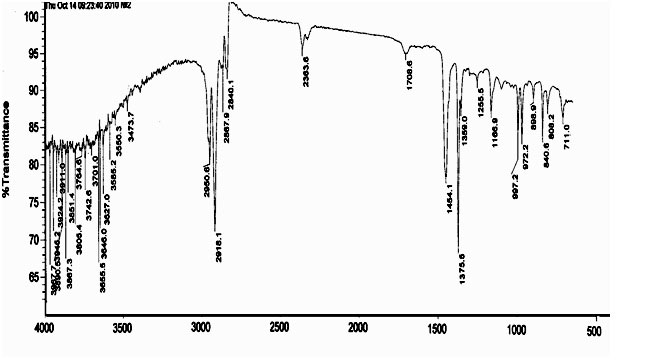
Pic.3. IR –Spectrum of OIPP
2. Polyfluoroalkylation of OIPP Using Octafluoropentylchlorosulphite
The reaction of OIPP with octafluoropentylchlorinesulphite was carried out in the following way: the particles of OIPP were slurred in chloroform at room temperature, then the solution was cooled to -10-(-5) °С and then in the presence of catalyst (dimethylformamide) the solution of octafluoropentylchlorine sulphite in chloroform was added. The reaction mass was kept while heating up to 40 °С for 1 hour. Then the obtained polyfluoroalkylated polypropylene (PFAPP) was washed with fresh portion of chloroform and dried at room temperature. The concentration of fluorine in PFAPP determined using method of burning the compound with further potentiometric titration using fluorine-silver electrode is equal to 15 %.
Besides that, if you compare the IR-spectra of OIPP and PFAPP (Pictures 3 and 4) you can see, that the absorption band in the field of valent oscillations of carbonyl group 1708 cm-1 for OIPP is shifting in IR-spectrum of PFAPP into the field of great wave numbers and is equal to 1713 cm-1. Besides that, in IR-spectrum sample of PFAPP a new absorption band appeared 1218 cm-1, which appearance is usually connected with valent oscillations of C-OC group – in ethers. At the same time the absorption in the field of valent oscillations of HO-group virtually disappears (3400–3600 cm-1), what can be explained by the participation of HO-group in the reaction of polyfluoroalkylation.
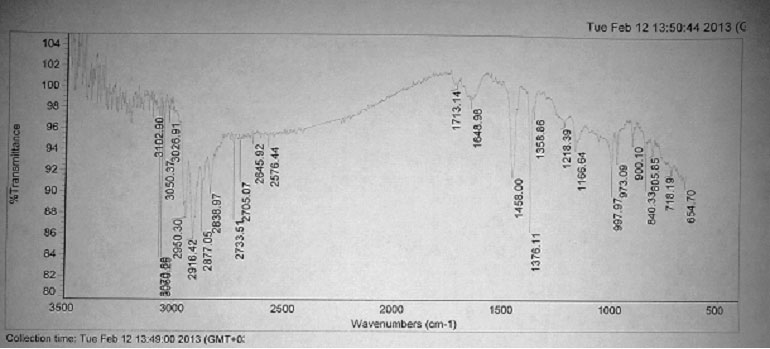
Pic. 4. FTIR Spectrum of PFAPP
Structural features of PAAIPP have been studied using method of electron microscopy (Pic. 5). You can see by comparing the photographs of surfaces of oxidized IPP and polyfluoroalkylated PFAIPP the character of surface particles changes greatly. Inhomogeneity of particles in the sample of the initial IPP, their variety of forms transforms into more homogeneous spheric structure of 100 µm sizes. It is explained by the fact, that there is the associative interaction of polar HO- and HOOC-groups in the initial IPP. There are no such intramolecular interactions in polyfluoroalkylated PFAIPP and particles acquire homogeneous spherical structure.
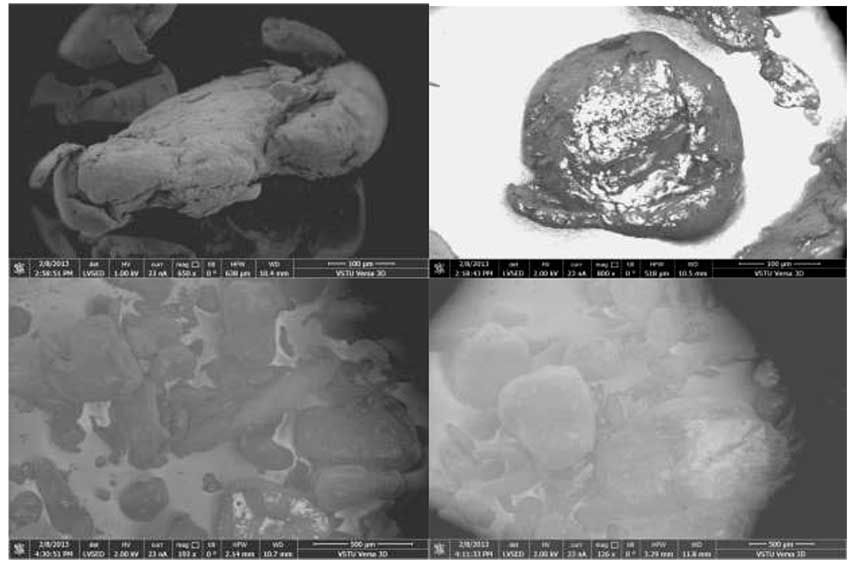
Pic. 5. Photographs of Surface of Oxidized (left) OIPP and Polyfluoroalkylated (right) PFAIPP
Conclusions
Surface modification of oxidized polypropylene using octafluoropentylchlorinesulphite with participation of polar HO- and HOOC-groups results in more homogeneous spherical structure for particles of 100 µm sizes possessing new set of properties, that opens wide perspectives for processing and application of polypropylene.
References
- I.P. Uklonskij, V.F Denisenkov, A.N. Il'in, S.N. Mineev, Yu.L. Bahmutov, L.M. Ivanova, Patent RU 2209204, MPK C 07 C31/38. Sposob polucheniya poliftorirovannyh spirtov Otkrytoe akcionernoe obshchestvo "Galogen" - 2002.
- A.I. Rahimov., O. V, Vostrikova, E. V.. Petrosyan. Osobennosti poverhnostnoj poliftoralkil modifikacii natrievoj soli karboksimetilcellyulozy// Ftornye zametki/ - 2013. - T.89., vyp. 4.
- A. I Rahimov., A. V. Nalesnaya, O. V. Vostrikova. Novyj metod sinteza poliftorirovannyh prostyh ehfirov .Zhurnal organicheskoj khimii / - 2004. - T. 74. , vyp. 4. - S. 1121.
- A. I. Rahimov, R. V. Fisechko. Reakciya poliftoralkilhlorsul'fitov s benzilovymi spirtami . Zhurnal obshchej khimii . - 2007. - T. 77, vyp. 10. - C. 1750-1751.
- A.I. Rahimov, R. V. Fisechko Novyj metod sinteza poliftoralkilciklo geksilovyh ehfirov. Zhurnal obshchej khimii. - 2008. - T. 78, vyp. 2. - S.338.
- A.I. Rahimov, A. V. Nalesnaya, R. V. Fisechko. -Osobennosti kataliza reakcii poliftoralkilhlorsul'fitov s predel'nymi odnoatomnymi spirtami . Zhurnal obshchej khimii / 2008. - T. 78, vyp. 11. - C. 1842-1848.
- A.I. Rahimov, A.V. Nalesnaya, R.V. Fisechko; Patent RU 2346926, MPK C 07 C43/192, C 07 C43/12, C 07 C43/225, C 07C 43/174. Sposob polucheniya prostyh poliftoralkilovyh ehfirov.GOU VPO VolgGTU. - 2009.
- A.I. Rahimov Sintez poliftoralkilhlorsul'fitov i novye reakcii s ih uchastiem // Zhurnal obshchej khimii - 2010. - T. 80, vyp. 8. - C. 1622-1641.
- A. I. Rahimov. Osobennosti zhidkofaznogo okisleniya o-, p-ksilolov v prisutstvii soedinenij s reakcionnosposobnymi CH-gruppami. Izvestiya VolgGTU : mezhvuz. sb. nauch. st. N 5 / VolgGTU.Volgograd, 2012. – (Seriya «Khimiya i tekhnologiya elementoorganicheskih monomerov i polimernyh materialov» ; vyp. 9). – S. 57–59.
- A.I.Rahimov Okislitel'naya destrukciya polipropilena v aromaticheskih uglevodorodah Izvestiya VolgGTU : mezhvuz. sb. nauch. st. N 2 /VolgGTU. – Volgograd, 2011. – (Seriya «Khimiya i tekhnologiya ehlementoorganicheskih monomerov i polimernyh materialov» ; vyp. 8). – S. 92–94.
- A. I. Rahimov. Khimiya i tekhnologiya organicheskih perekisnyh soedinenij /. – M.: Khimiya,1979. – C. 392.
- E. T. Denisov, Radikal'nye reakcii v tverdoj faze i mekhanizm okisleniya karbocepnyh polimerov .Uspekhi khimii. – 1978. – T. XLVII, vyp. 6. – S. 1090–1118.
- V. P. Nekhoroshev. Okislennyj atakticheskij polipropilen: poluchenie, svojstva i primenenie. ZhPKh. – 2000. – T. 73. – Vyp. 6. – S. 996–999.
- N. A. Rahimova. Osobennosti formirovaniya kompozicionnyh materialov na osnove okislennogo polipropilena i poliizocianata. Izvestiya VolgGTU : mezhvuz. sb. nauch. st.Volgograd, 2011. –Seriya «Khimiya i tekhnologiya elementoorganicheskih monomerov i polimernyh materialov» ;vyp. 8. – S. 88–91.
- A. I. Rahimov, A. Yu. Maryshev, N. A. Rahimova, M. A. Marysheva, D. V. Azarov. Stroitel'nye pokrytiya na osnove othodov polipropilena, Izvestiya VolgGTU : mezhvuz. sb. nauch. st. N 4 / VolgGTU. – Volgograd, 2013. –Seriya «Khimiya i tekhnologiya elementoorganicheskih monomerov i polimernyh materialov» ;vyp. 10. – C. 57–59.
- A.I.Rahimov.Kompozicii na osnove arilirovannogo gidroksil- soderzhashchego butadien-izoprenovogo oligomera PDI-1K// Izvestiya VolgGTU : mezhvuz. sb. nauch. st. N 5 / VolgGTU. – Volgograd, 2012. –Seriya «Khimiya i tekhnologiya elementoorganicheskih monomerov i polimernyh materialov» ; vyp. 9. S. 117–120.
- A. V. Nekhorosheva. Nauchnye osnovy metodov i sredstv bezopasnoj utilizacii othodov proizvodstva izotakticheskogo polipropilena: avtoref. dis. … d-ra tekh. nauk:25.00.36/. – SPb., 2009. – 30 s.
- N. A. Rahimova, A. I. Rahimov, E.V. Petrosyan, V.S. Avilova, A. Y. Maryshev, V.F. Zheltobryuhov. Strukturnye osobennosti poliftoralkilirovannogo funkcional'no zameshchennogo izotakticheskogo polipropilena. Izvestiya VolgGTU : mezhvuz. sb. nauch. st. N 4 /VolgGTU. – Volgograd, 2013. –Seriya «Khimiya i tekhnologiya elementoorganicheskih monomerov i polimernyh materialov» vyp.11,; N 19. – C. 102-105.
Recommended for publication by Prof. A. Rahimov
Fluorine Notes, 2015, 101, 1-2
