Received: February, 2015
DOI 10.17677/fn20714807.2015.02.05
Fluorine Notes, 2015, 99, 7-8
ACETOXYLATION OF 6,6,7,7,8,8,9,9-OCTAFLUOROPENTOXYPROP-1-ENE USING ROSIN ACIDS
L. M. Popova1, S.V. Vershilov2
1Saint-Petersburg State Technological University of Plant Polymers, 198095, 4 Ivan Chernykh
Str., Saint-Petersburg, Russia
e-mail: lorapopova@mail.ru
2State Enterprise S.V. Lebedev Research Institute of Synthetic Rubber (NIISK), 198035, Gapsalskaya Str. 1, St.-Petersburg, Russia
Abstract: Interaction of 6,6,7,7,8,8,9,9-octafluoropentoxyprop-1-ene and rosin acids under conditions of acidic catalysis results in formation of acetoxylation products. The process is going mainly against Markovnikov’s rule.
Keywords: Acetoxylation, 6,6,7,7,8,8,9,9-octafluoropentoxyprop-1-ene, rozin acids, 3-(2,2,3,3,4,4,5,5-octafluoropentoxy)propylabietate and dehydroabietate
Rosin acids are the main components of rosin. Formally rosin acids are referred to polyunsaturated monocarboxylic acids of phenanthrene row. The presence of carboxylic group as well as a couple (from 1 to 3) of double bonds in the structure of these compounds opens wide opportunities for obtaining of products used in many fields of industry. As a result of chemical modification by different methods (disproportioning, hydration, dimerization, diene synthesis, esterification) the application properties of rosin such as light and chemical resistance, increasing of melting point, crystallizing ability, compatibility with other materials, adhesiveness etc. greatly improve.
Products based on rosin and modified rosin are applied for sizing of paper and carton, in compositions of glues and paint-and-lacquer materials, they are also used for production of synthetic rubber, in electrotechnics and many other fields. Thus, adhesiveness, light and chemical resistance, absence of crystallization are important in the first place for pulp and paper industry and paint and varnish industry. The emulsifiers for synthetic rubber production using method of emulsion polymerization must not contain double bonds. Compounds for isolation of high-voltage cables must be highly isolation proof (moisture protection, high dielectric characteristics, crystallization free) within the whole period of articles’ exploitation [1].
It is known, that introduction of fluorine containing fragment into organic molecules in a number of case results in increasing of thermal and chemical resistance, generation of new practically valuable and sometimes unique characteristics [2].
The present work is devoted to studying of main patterns of interaction of abietic acid (AA) and dehydroabietic acid (I) mixture and 6,6,7,7,8,8,9,9-octafluoropentoxypropene-1 under conditions of acidic catalysis.
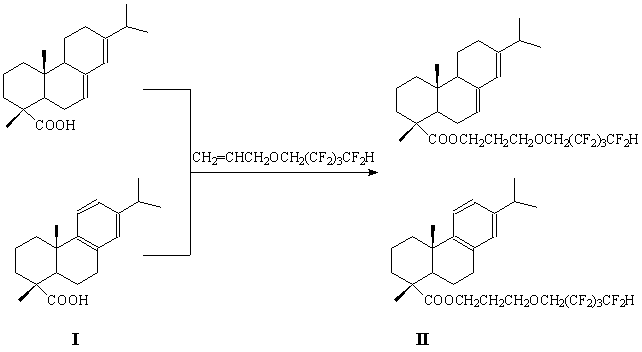
The reaction of 6,6,7,7,8,8,9,9-octafluoropentoxyprop-1-ene with rosin acids (I) was carried out while boiling in toluene or without solvent at catalysis of concentrated sulphuric acid or ZnCl2, at 110-115 or 140-145°С during 3, 10, 20 or 50 h. The control of reaction path was carried out using method of TLC (Thin Layer Chromatography). Upon the end of the process the reaction mass was diluted in diethyl ether (if necessary), washed through with distilled water to delete catalyst and dried with calcined sodium sulphate. After distillation of solvent and drying in vacuum over P2O5 amorphous light brown compounds were obtained (II) (yields are equal to 23-75%). Product melting point (II) is ranging 115 ÷ 140°С.
The composition and structure of mixture of 3-(2,2,3,3,4,4,5,5-octafluoropentoxy)propyl abietate and -dehydroabietate (II) were characterized by analytical data and data of IR, UV and NMR 1Н and 19F spectroscopy.
In NMR 1Н spectra the signals of diene protons of AA with chemical shifts of 5.80 (s., С7Н) and 5.40 (s., С14Н) ppm remain. However, we have found, that forming of ester was accompanied by dehydration of AA to dehydroabietic. This is confirmed by the increasing of intensity of signals of aromatic protons at 6.91 (s. С14Н), 7.02 (d. J 8 Hz, С12Н) and 7.19 (d. J 8 Hz, С11Н) ppm from 20% (in the initial mixture) up to 50% (by the end of the process).
The following signals of HCF2(CF2)3CH2-O-CH2CH2CH2-fragment protons confirm the formation of acetoxylation products: –ОС1Н2 (triplet) at 3.95 ppm, С2Н2 at 1.82 ppm, С3Н2 at 4.15 ppm, ОС5Н2 at 3.75 ppm (q) and end group –C9HF2 at 6.09 ppm in the form of triplets triplet with J=53 and J=6 Hz respectively. NMR 1H spectra analysis proves, that acetoxylation passes against the Markovnokov’s rule.
According to NMR 1Н spectra data the most full conversion reaches 90% when carrying out the reaction for 20 hours (reagents ratio 1 : 1, catalyst H2SO4, no solvent), in 10 hours the conversion is 70% (under the same conditions). When carrying out the reaction in boiling toluene (110-115°С) conversion went down to 20%. The decreasing of AA to alkene ratio down to 1 : 2 and increasing of reaction period up to 50 hours in toluene medium leads to complication of composition: along with acetoxylation products the signals belonging to the dimer structures (δ 5.24 ppm) appear.
In IR spectra of product (II) there are stripes νС-Н in the field of 3004-2870 cm-1, as well as νС=О were observed at 1695 cm-1 and virtually there are no stripes of valent oscillations of hydroxyl groups. Bands at 1190-1132 cm-1 correspond to valent oscillations of C-F bond.
Two maximum absorptions corresponding to π→π*-transfer at λmax 210 (lgε 3.61) and 243 nm (lgε 3.38) and confirming the presence of multiple bonds reveal themselves in UV-spectra of product (II) in alcohol solution.
In NMR 19F spectra of product (II) there are following resonance signals: C6F2CH2 at – 122.75 ppm, C7F2 at– 125.73 ppm, C8F2 at – 130.33 ppm, C9F2H in the form of doublet with chemical shift at – 137.29 ppm (J 42 Hz).
Experimental Part
UV spectra of alcohol solutions of compounds are obtained at spectrophotomether SF-2000 at compounds concentration equal to 10-4 mol/l, the thickness of absorbing layer is 1 cm.
NMR 1Н and 19F spectra have been registered using Bruker 500 of operational frequency of 500 MHz (470 MHz – for 19F), in solution CDCl3 outer standard - CCl3F.
IR spectra have been registered using Shimadzu IR Prestige-2. Measuring was carried out using glasses KBr (solutions in ССl4).
The reaction path and purity of initial and obtained compounds have been carried out using TLC method on SORBFIL discs, eluent: hexane –methylenechloride – acetone, (1 :1: 0.3).
Freshly distilled 6,6,7,7,8,8,9,9-octafluoropentoxyprop-1-ene (b. p 141 °С) synthesized analogously to methods [3] has been used as the initial one in the work.
The mixture of abietic and dehydroabietic acids (I), which isolation had been carried out according to method [4] from tall oil rosin SYLVAROS®85 (produced by Arizona Chemical) [5] was another subject of study.
The overall methods of 3-(1,1,2,2,3,3,4,4-octafluoropentoxy)propyl abietate (II) synthesis carrying out contained the following procedure: a mixture of rosin acids (I), 6,6,7,7,8,8,9,9-octafluoropentoxyprop-1-ene, toluene (or without solvent, 3-4 drops of concentrated sulphuric acid (or ZnCl2) was put into round bottom flask and heated up under reflux at 140-145°С. Upon finish of the procedure diethyl ether was added to the reaction mass, which was transferred into separating funnel, washed through using distilled water till reaching neutral reaction, the organic phase was dried using calcined Na2SO4, then it was filtered, left in air and dried in vacuum over Р2О5. The yield (II) accounted to 23 up to 75%, light brown amorphous compounds, softening point 120-126°С. IR spectra (film), cm-1: 2955, 2930, 2870 (νС-Н), 1695 (νС=О), 1456 (νСаr.), 1277 (νС-О-С), 1173-1132 (νСF). UV spectrum (EtOH), nm (lg ε): 210 (3.77); 243 (3.53). NMR 1Н spectrum, δ, ppm: 3.95 t (J=14 Hz), 4.07-4.17 m, 5.29-5.37 m, 5.40 (s. 1Н, С7Н), 5.80 (s. 1Н, С14Н), 6.09 t. t. (J=53 and J=6 Hz), 6.91 (s. 1Н, С14Н), 7.02 (d. 1Н, С12Н, J=9.6 Hz), 7.19 (d. 1Н, С11Н, J=9.6 Hz). NMR 19F spectrum, δ, ppm: 122.32 (2F, CF2CH2); 125.73 (2F, C7F2), 130.33 (2F, C8F2), 137.29 (d., 2F, CF2H, J=42 Hz).
Conclusions
It has been stated, that acetoxylation going against Markovnikov’s rule was the main direction of interaction of 6,6,7,7,8,8,9,9-octafluoropentoxyprop-1-ene and rosin acids. It has been found, that maximum conversion of reagents (up to 90%) was reached in 20 h at equimolar rate of reagents in absence of solvent and catalysis of concentrated H2SO4. The formation of acetoxylation products was accompanied by increase of aromatic component concentration in mixture.
It has been proved, that when carrying out the reaction between abietic acid and 6,6,7,7,8,8,9,9-octafluoropropoxyprop-1-ene at reagents ratio of 1 : 2 in toluene and catalysis of ZnCl2 during 50 h we observed the complicating of composition of the reaction mixture, in which dimer structures were found besides products of acetoxylation (about 30%) and disproportioning.
References
- Sandermann W. Naturharze Terpentinöltallöl. Cheme und Technologie / Sprinder Verlag: Berlin, 1960. 172 p.
- Fluorine compounds. Modern Technology and Application (In Russian). / N. Ishikawa (Ed.) : Мir Publ., 1984, 592 p.
- M. Hudlicky. Chemistry of Organic Fluorine Compounds. Pergamon Press; First Edition, 1961. 536 p.
- Lazur'evskii, G. V., Terent'eva, I. V., Shamshurin, A. A. Practical Work in the Chemistry of Natural Compounds, Moscow, “Vysshaya Shkola” Publ., 1966. 158 p.
- SYLVAROS®85 Product Data Sheet (PDS): http://www.arizonachemical.com/wp-content/uploads/SYLVAROS®-85.pdf
Recommended for publication by V. Kornilov
Fluorine Notes, 2015, 99, 7-8
