Received: November, 2014
DOI 10.17677/fn20714807.2015.01.01
Fluorine Notes, 2015, 98, 3-4
Synthesis fluorocontaining derivatives of pyrazolo[3,4-d]pyrimidines.
Message 2. Synthesis
of fluorocontaining substituted amides of 5-(fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic
acid and substituted 5-aryl-6-aryl-1-phenyl-1,5-dihydropyrazolo-[3,4-d]pyrimidine-4-ones.
M.E.Zhidkov, A.V.Kutkin, A.F.Eleev
State Research Institute of Organic Chemistry & Technology
23, Shosse Entuziastov, 111024
Moscow, Russian Federation
e-mail: dir@gosniiokht.ru
Abstract: Synthesized according to the method developed by us earlier pyrazolo[3,4-d] [1,3]oxazine involved in the reaction with various primary and secondary amines to form amides of 5-(fluorobenzoylamino)-1-phenyl-1H-pyrazole-4-carboxylic acid. Synthesized previously benzamides of pyrazole-4-carboxylic acid was subjected to high temperature cyclization with the formation of substituted 5-aryl-6-aryl-1-phenyl-1,5-dihydropyrazolo-[3,4-d]pyrimidine-4-ones.
Keywords: pyrazolo[3,4-d][1,3]oxazine, dihydropyrazolo-[3,4-d]pyrimidine-4-one, benzamide, thin layer chromatography, 1H NMR spectra.
As was shown in our previous article [1] derivatives of pyrazolo[3,4-d]pyrimidines currently presents a fairly extensive number of examples of biologically active substances with antimicrobial [2,3], antiviral [4,5], antiallergic [6], antihypotensives [7-9], antitumor [10], anti-inflammatory [11] and analgesic properties [12].
Synthesized by us earlier [1] according to the developed method derivatives of pyrazolo[3,4-d] [1,3]oxazin-4-one with general formula:
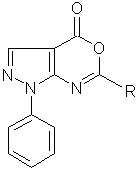
where R represents a 2,3 and 4 - fluorosubstituted phenyl fragment are highly reactive compounds. In addition to the previously discussed interaction with various substituted anilines we have implemented the interaction of substituted pyrazolo[3,4-d] [1,3]oxazin-4-ones with various primary and secondary amines that can be illustrated by the reaction of obtaining 5-(3-perbenzoate)-1-phenyl-1H-pyrazole-4-carboxylic acid morpholide(1a):
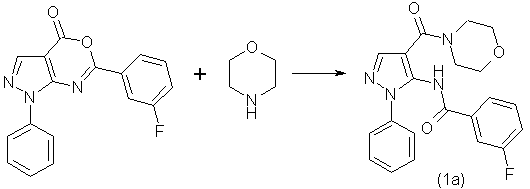
The reaction was carried out by mixing equimolar amounts of 6-(3-fluorophenyl)-1-phenyl-1H-pyrazolo-[3,4-d][1,3]oxazin-4-one
and morpholine and in contrast to the reaction with aniline was accompanied by a strong exothermic
effect.
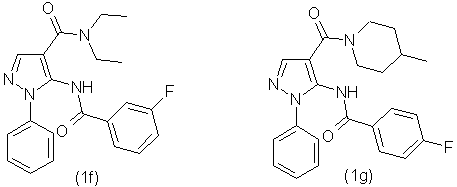
By the similar method using different amines, we have obtained the following
compounds: using 2-furfurylamine was obtained compound (1b), using 3-methylpiperidine was
obtained compound (1c), using 3-pyridylmethylamine was obtained compound (1d), using N-ethylpiperazine
was obtained compound (1e), using N,N-diethylamine was obtained the compound (1f), using
4-methylpiperidine was obtained the compound(1g). The synthesized compounds have the following
formulas:
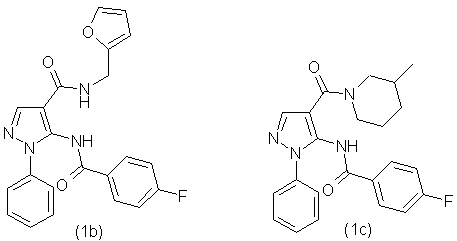
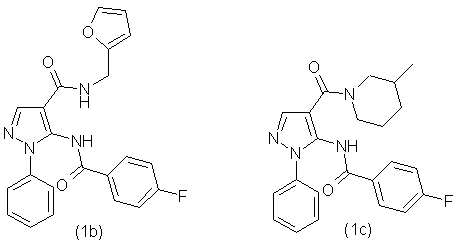
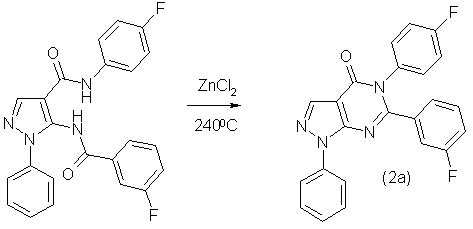
Synthesized according to the proposed method [1] substituted 5-(fluorobenzoylamino) arylamides of 1-phenyl-1H-pyrazole-4-carboxylic acid were subjected to thermal cyclization by using as a catalyst anhydrous zinc chloride. This process can be illustrated at the example of a 5-(4'-fluorophenyl)-6-(3''-fluorophenyl)-1-phenyl-1,5-dihydropyrazolo-[3,4-d]pyrimidin-4-one (2a):
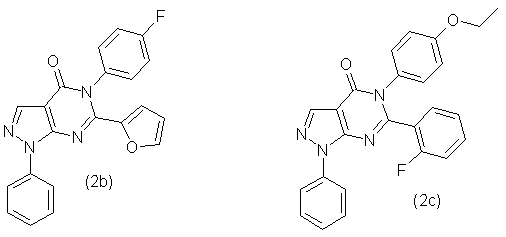
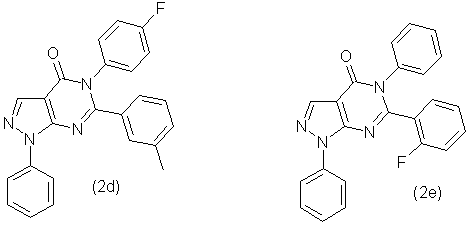
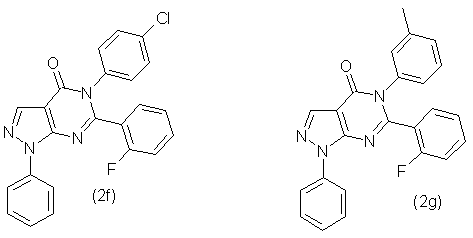
Similarly, in accordance with this method were synthesized compounds 2b, 2c, 2d, 2e, 2f and 2g, which has the following formula:
Experimental
1H NMR spectra (external reference TMS) were obtained in DMSO-d6 on the device Bruker AM-400 (400 MHz), melting temperatures of the substances were defined on the device Mettler FP5. Monitoring the progress of the reaction and individuality of the obtained compounds was carried out by TLC on plates Silufol UV-254 in the system of toluene-acetone 4:1.
5-(3-Fluorobenzoylamino)-1-phenyl-1Н-pyrazolo-4-carboxylic acid morpholide (1a).
In a three-neck flask on 25 ml, equipped with condenser were placed 1 g (0,003 mol) 6-(3-fluorophenyl)-1-phenyl-1Н-pyrazolo-[3,4-d][1,3]oxazin-4-one and 2 ml of freshly distilled morpholine. The reaction takes place with a significant thermal effect and ends within 15 minutes. Then, to the reaction mixture was added 10 ml of ethyl alcohol. While cooling in an ice bath falls white precipitate, which is filtered off, washed with ethyl alcohol and dried first on the filter and then in vacuum. Thus was prepared 0,98 g (76,18%) 5-(3-Fluorobenzoylamino)-1-phenyl-1Н-pyrazolo-4-carboxylic acid morpholide as a white crystals with mp. 170 – 173 °С. Rf 0,35 (toluene-acetone 4:1). NMR spectrum 1Н (20% in СDCl3), δ, m.d.: 3,55 (8H, s, morph), 7,40 (1H, m, С6Н5), 7,50 (3Н, m, С6Н5 ), 7,58 (3Н, m, С6Н5), 7,65 (1H, m, С6Н5), 7,73 (1Н, m, С6Н5), 7,94 (1Н, s, -СН=), 10,40 (1Н, s, NH). Found, %: C 63,81; Н 4,88; N 14,55; F 4,73. С21H19FN4O3 Calculated, %: C 63,95; Н 4,86; N 14,21; F 4,82.
According to the similar method were synthesized the following compounds:
-5-(4-Fluorobenzoylamino)-1-phenyl-1Н-pyrazolo-4-carboxylic acid (2-furfuryl)amide (1b) with output 89,0% as the white powder with mp.180-183 °С. NMR spectrum 1Н (20% in СDCl3), δ, m.d.: 4,41 (2H, d, СН2), 6,25(1Н, d, С6Н5), 6,38 (1Н, m, С6Н5), 7,38 (3Н, m, С6Н5), 7,45 (2Н, t, С6Н5), 7,95 (2Н, m, С6Н5), 8,26 (1Н, s, -СН=), 8,61 (1Н, t, NH), 10,42 (1Н, s, NH). Found, %: C 65,57; Н 4,19; N 13,59; F 4,83. С22H17FN4O3 Calculated, %: C 65,34; Н 4,24; N 13,85; F 4,70.
-5-(4-Fluorobenzoylamino)-1-phenyl-1Н-pyrazolo-4-carboxylic acid (3-piperidyl)amide (1c) with output 81,3% as the white powder with mp.192-195 °С. NMR spectrum 1Н (20% in СDCl3), δ, m.d.: 0,80 (2Н, d, СН3), 1,10 (1Н, m, СН), 1,50 (3Н, m, СН), 1,70 (1Н, d, СН2), 2,60 (1Н, m, СН), 3,00 (1Н, m, СН), 3,80 (1Н, m, СН), 4,30 (1Н, m, СН), 7,35 (3Н, m, С6Н5), 7,45 (2Н, t, С6Н5), 7,55 (2Н, d, С6Н5), 7,85 (1Н, s, -СН=), 7,90 (2Н, m, С6Н5), 10,50 (1Н, s, NH). Found, %: C 68,02; Н 5,59; N 13,83; F 4,51. С23H13FN4O2 Calculated, %: C 67,97; Н 5,70; N 13,78; F 4,67.
-5-(4-Fluorobenzoylamino)-1-phenyl-1Н-pyrazolo-4-carboxylic acid (3-pyridyl)methylamide (1d) with output 78,1% as the white powder with mp.180-185 °С. NMR spectrum 1Н (20% in СDCl3), δ, m.d.: 4,45 (2Н, d, СН2), 7,35 (4Н, m, С6Н5), 7,49 (2Н, t, С6Н5), 7,55 (2Н, d, С6Н5), 7,70 (1Н, d, С6Н5), 7,95 (2Н, m, С6Н5), 8,26 (1Н, s, -СН=), 8,48 (1Н, d, С6Н5), 8,53 (1Н, m, С6Н5), 8,78 (1Н, t, NH), 10,43 (1Н, s, NH). Found, %: C 66,37; Н 4,29; N 16,91; F 4,52. С23H23FN4O2 Calculated, %: C 66,50; Н 4,37; N 16,86; F 4,37.
-5-(4-Fluorobenzoylamino)-1-phenyl-1Н-pyrazolo-4-carboxylic acid (N-ethylpiperazine)amide (1e) with output 87,7% as the white powder with mp.152-155 °С. NMR spectrum 1Н (20% in СDCl3), δ, m.d.: 0,95 (3Н, t, СН3), 2,28 (6Н, m, СН2), 3,50 (4Н, s, СН2), 7,36 (3Н, q, С6Н5), 7,46 (2Н, t, С6Н5), 7,57 (2Н, d, С6Н5), 7,90 (1Н, s, -СН=), 7,94 (2Н, m, С6Н5), 10,56 (1Н, s, NH). Found, %: C 65,81; Н 5,59; N 16,71; F 4,47. С23H24FN5O2 Calculated, %: C 65,54; Н 5,74; N 16,62; F 4,51.
-5-(3-Fluorobenzoylamino)-1-phenyl-1Н-pyrazolo-4-carboxylic acid (N,N-diethylamide (1f) with output 81,2% as the white powder with mp.180-183 °С. NMR spectrum 1Н (20% in СDCl3), δ, m.d.: 1,08 (6Н, s, СН3), 3,30 (4Н, s, СН2), 7,39 (1Н, t, С6Н5), 7,49 (3Н, m, С6Н5), 7,60 (4Н, m, С6Н5), 7,70 ((1Н, m, С6Н5), 7,92 (1Н, s, -СН=), 10,40 (1Н, s, NH). Found, %: C 66,17; Н 5,49; N 14,81; F 4,77. С21H21FN4O2 Calculated, %: C 66,30; Н 5,56; N 14,73; F 4,99.
-5-(4-Fluorobenzoylamino)-1-phenyl-1Н-pyrazolo-4-carboxylic acid (4-piperidyl)amide (1g) with output 90,1% as the white powder with mp.175-177 °С. NMR spectrum 1Н (20% in СDCl3), δ, m.d.: 0,85 (3Н, d, СН3), 1,00 (2Н, m, СН), 1,60 (3Н, m, СН), 2,80 (2Н, m, СН), 3,85 (1Н, m, СН), 4,35 (1Н, m, СН), 7,37 (3Н, q, С6Н5), 7,48 (2Н, t, С6Н5), 7,57 (2Н, d, С6Н5), 7,88 (1Н, s, -СН=), 7,92 (2Н, m, С6Н5), 10,55 (1Н, s, NH). Found, %: C 66,41; Н 4,23; N 16,89; F 4,42. С23H23FN4O2 Calculated, %: C 66,50; Н 4,37; N 16,86; F 4,37.
5-(4'-Fluorophenyl)-6-(3''-fluorophenyl)-1-phenyl-1,5-dihydropyrazolo-[3,4-d]pyrimidin-4-one (2a)
In a three-neck flask on 10 ml, equipped with condenser, mechanical stirrer and thermometer were placed 1,15 g (0,0028 mol) 5-(3-fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid (4-fluorophenyl) amide and several crystals of anhydrous zinc chloride. The reaction mixture was heated to 220 – 240 °С and kept at this temperature until the termination of allocation of water (about 30 minutes). Then the reaction mass is then cooled to room temperature, add 5 ml of ethanol and heated to boiling for 5 minutes. The resulting dark colored solution is cooled in an ice bath, the precipitated precipitate is filtered on the filter, washed with ethyl alcohol and dried in the beginning on the filter and then in vacuum.
Thus was prepared 0,79 g (71,27%) 5-(4'-Fluorophenyl)-6-(3''-fluoro-phenyl)-1-phenyl-1,5-dihydropyrazolo-[3,4-d]pyrimidin-4-one as the white powder with mp. 210 – 213 °С. Rf 0,41 (toluene-acetone 4:1). NMR spectrum 1Н (20% in СDCl3), δ, m.d.: 7,17 (1Н,t, С6Н5), 7,45 (3Н, m, С6Н5), 7,58 (3Н, m, С6Н5), 7,68 (3Н, m, С6Н5), 7,70 (1Н, d, С6Н5), 8,42 (1Н, s, -СН=). Found, %: C 66,27; Н 3,81; N 13,42; F 9,12. C23H16F2N4O2 Calculated, %: C 66,03; Н 3,85; N 13,39; F 9,08.
According to the similar method were synthesized the following compounds:
- 5-(4'-Fluorophenyl)-6-(2''-furoyl)-1-phenyl-1,5-dihydropyrazolo-[3,4-d]pyrimidin-4-one (2b) with output 76,7 % as the white powder with mp. 230-235 °С. NMR spectrum 1Н (20% in СDCl3), δ, m.d.: 5,88 (1Н, d, С6Н5), 6,52 (1Н, m, С6Н5), 7,43 (3Н, m, С6Н5), 7,52 (2Н, m, С6Н5), 7,60 (2Н, t, С6Н5), 7,85 (1Н, d, С6Н5), 8,18 (2Н, d, С6Н5), 8,43 (1Н, s, -СН=). Found, %: C 67,69; Н 3,61; N 15,02; F 4,99. С21H13FN4O2 Calculated, %: C 67,74; Н 3,52; N 15,05; F 5,10.
- 5-(4'-Ethoxyphenyl)-6-(2''-fluorophenyl)-1-phenyl-1,5-dihydropyrazolo-[3,4-d]pyrimidin-4-one (2c) with output 79,8 % as the white powder with mp. 240-245 °С. NMR spectrum 1Н (20% in СDCl3), δ, m.d.: 1,28 (3Н, t, СН3), 3,94 (2Н, q, СН2), 6,83 (2Н, d, С6Н5), 7,16 (4Н, m, С6Н5), 7,39 (2Н, m, С6Н5), 7,55 (3Н, t, С6Н5), 8,02 (2Н, d, С6Н5), 8,50 (1Н, s, -СН=). Found, %: C 67,69; Н 3,61; N 15,02; F 4,99. С25H19FN4O2 Calculated, %: C 70,41; Н 4,49; N 13,14; F 4,45.
- 5-(4'-Fluorophenyl)-6-(3''-methylphenyl)-1-phenyl-1,5-dihydropyrazolo-[3,4-d]pyrimidin-4-one (2d) with output 82,9 % as the grey powder with mp. 165-170 °С. NMR spectrum 1Н (20% in СDCl3), δ, m.d.: 2,20 (3Н, s, СН3), 7,16 (6Н, m, С6Н5), 7,38 (3Н, m, С6Н5), 7,56 (2Н, t, С6Н5), 8,08 (2Н, d, С6Н5), 8,48 (1Н, c, -СН=). Found, %: C 72,59; Н 4,34; N 14,28; F 4,71. С24H17FN4O Calculated, %: C 72,72; Н 4,32; N 14,13; F 4,79.
- 5-Phenyl-6-(2''-fluorophenyl)-1-phenyl-1,5-dihydropyrazolo-[3,4-d]-pyrimidin-4-one (2e) with output 85,2 % as the grey powder with mp. 245-250 °С. NMR spectrum 1Н (20% in СDCl3), δ, m.d.: 7,06 (1Н, t, С6Н5), 7,14 (1Н, t, С6Н5), 7,30 (5Н, m, С6Н5), 7,42 (1Н, t, С6Н5), 7,58 (4Н, t, С6Н5), 8,02 (2Н, d, С6Н5), 8,52 (1Н, s, -СН=). Found, %: C 72,31; Н 4,02; N 14,58; F 4,91. С23H15FN4O Calculated, %: C 72,24; Н 3,95; N 14,65; F 4,97.
- 5-(4'-Chlorophenyl) -6-(2''-fluorophenyl)-1-phenyl-1,5-dihydropyrazolo-[3,4-d]-pyrimidin-4-one (2f) with output 91,2 % as the grey powder with mp. 233-235 °С. NMR spectrum 1Н (20% in СDCl3), δ, m.d.: 7,12 (1Н, m, С6Н5), 7,20 (1Н, t, С6Н5), 7,40 (8Н, м, С6Н5), 7,60 (2Н, m, С6Н5), 8,00 (1Н, m, С6Н5), 8,55 (1Н, s, -СН=). Found, %: C 66,19; Н 3,42; N 13,52; F 4,52. С23H14ClFN4O Calculated, %: C 66,27; Н 3,39; N 13,44; Cl 8,51; F 4,56.
- 5-(3'-метилфенил) -6-(2''-fluorophenyl)-1-phenyl-1,5-dihydropyrazolo-[3,4-d]-pyrimidin-4-one (2g) with output 83,2 % as the grey powder with mp. 241-243 °С. NMR spectrum 1Н (20% in СDCl3), δ, m.d.: 2,20 (3Н, s, СН3), 7,15 (7Н, m, С6Н5), 7,35 (3Н, m, С6Н5), 7,55 (2Н, m, С6Н5), 8,00 (1Н, d, С6Н5), 8,45 (1Н, с, -СН=). Found, %: C 72,59; Н 4,41; N 14,22; F 4,83. С24H17FN4O Calculated, %: 72,72; Н 4,32; N 14,13; F 4,79.
References
- M.E.Zhidkov, A.V.Kutkin, A.F.Eleev, Fluorine notes, 4 (95), 2014.
- S. V. Nielsen, E. B. Pedersen, Chem. Scr, 23, 1984, p. 240-244.
- M. M. Ghorab, Acta Pharmaceutica (Zagreb), 50, 2000, p. 93-110.
- E. R. Bendary, F. A. Badria, Arch. Pharm., 333, p. 99-103.
- C. Jyh-Haur, S. Kak-Shan, H. Tsu-An, T. Chia-Liang, et al., Bioorg. Med. Chem. Lett., 14, 2004, p. 2519-2531.
- P. P. Gupta, R. C. Srimal, K. Avasthi, T. Chandra, D. S. Bhakuni, Indian J. Exp. Biol., 33, 1995, p. 38-40.
- S. A. El-Feky, Z. K. Abd el-Samii, Pharmazie, 51, 1996, p. 540-543.
- G. Szilagyi, E. Kasztreiner, L. Tardos, E. Kosa, L. Jaszlits, G. Cseh, et al., Ger. Offen. # 2,825, 906 (Cl. CO 7D 403/04), (1979), Hung. Appl. 1, 373, 1977; Chem. Abstr., 90, 152224x (1979).
- B. Dumaıˆtre, N. Dodic, J. Med. Chem., 39,1996, р. 1635 – 1644.
- M. M. Ghorab, F. A. Ragab, E. Noaman, H. I. Heiba, S. A. Aboulmagd, Arzneimittelforschung, 59 (2), 2009, р. 96–103.
- I. Devesa, M. J. Alcaraz, A. Riguera, M. L. Ferrandis, Eur. J. Pharm., 488, 2004, p. 225-230.
- J. W. Marsico, J. P. Joseph, US Patet # 3,864,359 (Cl. 260–310R; CO 7d), 4 February 1975, Appl. 248, 990, 1 May 1972; 6 pp; Chem. Abstr., 82, 170936v (1975).
- M. Chauhan, R. Kumar, Bioor.& Med.Chem., 21, 2013, p. 5657-5668.
Recommended for publication by Prof. A. F. Eleev
Fluorine Notes, 2015, 98, 3-4
