Received: October, 2012
Fluorine Notes, 2014, 97, 1-2
Chemistry and Technology of Polyfluorinated Organic Compounds Based on New Aggression Resistant Catalysts
Vladimir Y. Zakharov
State Educational Institution of Higher Professional Education
"Vyatka State University", 610000,
Kirov, Moskovskaya str. 36, Russia
e-mail: zakhar.05@mail.ru
Abstract: Scientific principles of selection of effective catalysts of direct gas-phase fluorination of organic compounds have been developed. The use of created catalysts improve existing and allow to develop the new technologies of polyfluorinated organic products.
Keywords: Organofluorine compounds, fluorine, cobolt trifluoride, isomerization, heterogeneous catalyst, catalytic fluorination.
(End of the article)
3.3. Oxidation of Tetrafluoroethylene; Obtaining of Carbonyldifluoride
Carbonyldifluoride (CF) is a base for the synthesis of a whole range of oxygen containing fluoroorganic compounds, particularly, perfluoromethylperfluorovinyl ether /138/. One of the promising ways of CF obtaining is an oxidation of TFE using molecular oxygen:
C2F4 + O2 → 2COF2 (– 620 kJ/mole)
Gas phase interaction of TFE and oxygen is accompanied by isolation of significant amounts of heat and is of explosive character; that fact in a significant way hampers carrying out of the process on both laboratory and industrial scales.
Using the inert diluent is one of the possible ways to prevent the explosive reaction. CF/241/ is applied as diluent – 20-100 moles per oxygen mole; the process is carried out at 470-720 K, contact period of 1-10 sec and mole ratio of TFE:O2, equal to 1. It is being noticed /241,242/, that CF is the main product of such high temperature gas-phase oxidation; thus, at 694 K the conversion of TFE amounted to 99,5%, and the yield of CF – 98% /241/. Difluorochlomethane and trifluorotrichloethane are also described as inert diluent at gas phase oxidation of TFE using oxygen /243/.
We shall notice, that though carrying out of the process adding low-boiling inert diluents increases the explosion safety of oxidation it in a significant way impeds the isolation of target product; recycle of CF as inert diluent can also be accompanied by its losses. Here developed by us explosion proof method of oxidation of fluororganic compounds in hydrogen-air flame is unacceptable/244, 245/, because the presence of water results in hydrolysis and loss of part of target product.
Above we have shown high efficiency of α-Al2O3as a carrier for catalysts of a wide range of thermal transformations of polyfluorinated organic compounds In this connection we have studied an opportunity of CF obtaining by TFE oxidation using undiluted oxygen in the layer of α-Al2O3, modified by nickel, copper, iron, cobalt oxides. The results are listed in Table 36; here for you to compare we have listed data obtained during TFE oxidation in the layer of quartz packing.
Listed results indicate a significant impact of catalyst nature on the rate of oxidation. Thus, at quartz packing the oxidation of TFE start 460 – 470 K, while using α-Al2O3 modified by copper, nickel and iron, and also at unmodified α-Al2O3the temperature of reaction ignition/firing lowers to 350-360 K. At that the oxidation passes without induction period and is characterized by virtually numerical forming of CF.
Cobalt modified α-Al2O3is of high activity; in this case, TFE oxidation passes all the time at the initial temperature in the reactor which is as low as 323 K (experiment 17, Table 36).
Lowering of oxidation ignition temperature after operation is an interesting feature of all studied catalysts. The analysis of composition of heterogeneous contacts (after thermal treatment using mixture of TFE and O2) demonstrated the presence of perfluorinated polyperoxides (PP) on their surface, which apparently are the initiators of oxidation and cause the increase of catalytic systems (Table 37).
Table 36. Tetrafluoroethylene Oxidation Using Oxygen ( reagents’volume rate of input is 50 hour-1, mole ratio TFE:О2=1).
|
# |
Catalyst |
T, K |
Reaction Gases Composition1, Volume % |
|||||
|
CF4 |
CO2 |
COF2 |
C2F4 |
CF3COF |
Others |
|||
|
1 |
SiO2 (Quartz) |
453 |
0,01 |
0,02 |
0,80 |
99,12 |
0,04 |
0,02 |
|
2 |
Same |
463 |
0,01 |
0,04 |
46,38 |
52,04 |
0,30 |
1,24 |
|
3 |
-«- |
473 |
<0,01 |
0,08 |
95,31 |
2,42 |
0,84 |
1,35 |
|
4 |
α-Al2O3 |
343 |
<0,01 |
0,02 |
0,74 |
99,20 |
0,02 |
0,02 |
|
5 |
Same |
353 |
<0,01 |
0,04 |
89,32 |
9,22 |
0,62 |
0,80 |
|
6 |
-«- |
363 |
0,53 |
0,80 |
95,62 |
0,82 |
1,52 |
0,71 |
|
7 |
Ni/α-Al2O3 |
343 |
<0,01 |
0,02 |
1,94 |
97,71 |
0,02 |
0,31 |
|
8 |
Same |
353 |
<0,01 |
0,08 |
92,46 |
6,22 |
0,80 |
0,44 |
|
9 |
-«- |
363 |
<0,01 |
0,08 |
96,98 |
1,25 |
1,16 |
0,53 |
|
10 |
Cu/ α-Al2O3 |
343 |
0,01 |
0,02 |
0,68 |
99,02 |
0,02 |
0,28 |
|
11 |
Same |
353 |
0,17 |
0,36 |
72,81 |
19,24 |
6,98 |
0,44 |
|
12 |
-«- |
363 |
0,26 |
0,40 |
86,30 |
5,38 |
7,12 |
0,54 |
|
13 |
Fe/ α-Al2O3 |
343 |
0,02 |
0,04 |
0,98 |
98,62 |
0,16 |
0,18 |
|
14 |
Same |
353 |
0,12 |
0,26 |
87,87 |
9,62 |
1,60 |
0,53 |
|
15 |
-«- |
373 |
0,24 |
0,35 |
94,94 |
0,80 |
3,05 |
0,62 |
|
16 |
Cо/ α-Al2O3 |
313 |
<0,01 |
0,04 |
0,09 |
99,77 |
0,02 |
0,08 |
|
17 |
Same |
323 |
<0,01 |
0,08 |
98,04 |
0,10 |
1,68 |
0,10 |
|
18 |
-«- |
333 |
<0,01 |
0,08 |
98,17 |
<0,01 |
1,62 |
0,13 |
1Without taking into account unreacted oxygen.
Developed catalyst of Cо/α-Al2O3is active even at room temperature after prolonged storage at the open air (experiment 6, Table 37). This circumstance along with the absence of induction period is quite important from the practical point of view and it also increases explosion safety of the process, because it excludes conversion of reagents and corresponding appearing of explosives (in communications) even at switching off the reactor’s heating.
Table 37. The impact of thermal (473 K) treatment using mixture of tetrafluoroethylene and oxygen ( treatment period - 24 hours).
|
# |
Catalyst |
Temperature of Equimolar Mixture of TFE and О2 Ignition |
Concentration of PP, gram-equivalent per 100 g of catalyst, * 103 |
|
|
For Fresh Sample |
For Treated Sample |
|||
|
1 |
Quartz |
460-470 |
420-430 |
- |
|
2 |
α-Al2O3 |
350-360 |
320-330 |
13,0 |
|
3 |
Ni/α-Al2O3 |
350-360 |
320-330 |
1,7 |
|
4 |
Cu/α-Al2O3 |
350-360 |
320-330 |
0,3 |
|
5 |
Fe/ α-Al2O3 |
350-360 |
320-330 |
0,3 |
|
6 |
Cо/ α-Al2O3 |
320-325 |
<298 |
0,4 |
The peculiar feature of catalysts based α-Al2O3is, as tests proved, their high stability: operation for 400 doesn’t lead to lowering of conversion degree, decrease of mechanical durability of samples and is characterized by virtually quantitive yield of CF. According to the results of this part of work the initial data for projecting of CF technology by TFE oxidation using oxygen at catalyst of cobalt oxide/α-Al2O3 (mark KGN-I).
We shall notice, that results of this part of the work described in /246/ provoked the studies regarding selecting of heterogeneous catalysts of TFE oxidation /142/ Catalysts, which are PP on the surface of solid carrier – activated charcoal, silica gel or alumogel, were created as a result of those works, they are of high activity even under mild conditions. The drawback of those catalysts is their gradual decay at prolonged operation, which is caused by use of carriers unstable under oxidizing fluorinating media.
Thus, based on summoning the results of study of a wide class of thermal gas phase transformation of polyfluorinated organic compounds it is stated, that α-Al2O3is a universal aggression resistant carrier for effective catalysts of those processes. Based on α-Al2O3a universal effective catalyst of direct fluorination of polyfluorinated organic compounds (NiF2/α-Al2O3)was created, stable catalysts of selective dechlorination of 1,2-dichlorohexafluoropropane to HFP by hydrogen (Ni/α-Al2O3), of 1,2-difluorotetrachloroethane to 1,2-difluordichloroethylene (α-Al2O3; Ni/α-Al2O3), of 1,1,2-trifluorotrichloroethane to trifluorochloroethylene (Cu, BaO/α-Al2O3), a low-temperature catalyst of directed oxidation of TFE to CF was developed (cobalt oxide/α-Al2O3).
4. Catalysis of Polyfluorinated Organic Compounds’ Nucleophilic Thermal Reactions Using activated charcoal
Activated charcoal (AC) is quite stable to the influence of fluoroorganic compounds, as well as hydrogen fluoride and hydrogen chloride and it is not decomposed by those aggressive products even at most long contacting period and high temperature. That creates requisites for using of activated charcoal as catalyst of thermal transformations of polyfluorinated organic compounds.
Activated charcoal is used as a rule as a carrier for preparing electrophilic catalysts of gas phase fluorination by hydrogen fluoride, disproportioning or isomerization of fluorocarbons. Thus, activated charcoal modified by oxides, oxifluorides, fluorides or chlorides of chrome, molibdene, iron, aluminium or nickel is used during hydrogen fluoride fluorination of chloroform /247,248/, 1,1,2-trifluoro-2-chloroethyldichloromethyl ether /249/, β-trichloromethyl pyridine /250/, methyl alcohol /251/; chrome oxide applied onto activated charcoal according to /252/, is an effective catalyst of disproportioning of 1,1,1-trifluorotrichloroethane and 1,1,1-trifluoro-2-chloroethane and activated charcoal modified by salts of chrome, iron, molibdene and copper –a catalyst of isomerization of 1,1-dichlorotetrafluoroethane /253/.
In /254-256/ you can find the description of use of activated charcoal as a carrier for alkali metals fluorides – nucleophilic catalysts of isomerization of tetrafluoroethylene /254/ and hexafluoropropylene /255, 256/ oxides. In all those works a modified activated charcoal is described, that hampers estimating the nature of catalytic impactof carbon surface itself due to tampering influence of applied active component.
A quite smaller number of works is devoted to the catalysis of reactions of fluororganic compounds at unmodified carbon. Thus, prolonged contacting of activated charcoal with hexafluoropropyleneoxide at 260-300 K (40-70 hours) or with tetrafluoroethyleneoxide at 200-310 K /257-262/ results in formation of corresponding oligomeric perfluoropolyethers with wide molecular-massive distribution. In /263/ you can find a description of 2-perfluoromethylperfluoropentene-2 at heating up of hexafluoropropylene over activated charcoal. It is typical, that all those reactions – the oligomerization of tetrafluoroethyleneoxide, hexafluoropropyleneoxide and hexafluoropropylene are typical nucleophilic processes, which are intensified by main heterogeneous contacts – halogenides /264-267/ and perfluoroalkooxides of alkali metals /268/, univalent copper salts /269,270/, salts of quatemary ammonium and phosphonium bases /266/. This provides grounds to suppose, that an unmodified activated charcoal is also a main catalyst of nucleophilic type.
Activated carbon is widely used in chemical industry as adsorbent and catalyst, particularly, of oxidation of sulphur and hydrogen sulfide dioxide, of synthesis of phosphogen and sulphuryl chloride, of chlorineciane trimerization /271/. Taking into account the availability of activated charcoal, its high specific surface and mechanical strength it was seen worth studying the catalytic properties of this contact in nucleophilic reactions of polyfluorinated organic compounds more closely, a number of which, as a preliminary analytical review showed, is intensified by active centers of carbon surface. Gas-phase reactions were of a specific interest here. Diffusion limitations belonging to catalysis at close-porous contacts, in particular, at activated charcoal are less typical for them.
We have studied gas-phase isomerization of hexafluoropropyleneoxide (HFPO) to perfluoropropionylfluoride (PFPrF) as a model reaction for estimating nucleophilic catalytic activity of carbon, this reaction is intensified by main catalysts – fluorides of alkali metals /255, 272/, secondary and tertiary amines (aliphatic and aromatic, for example, trimethylamine, triethylamine, dimethylamine, pyridine /273-275/), and also tertiary amines, for example, dimethylformamide, dimethylacetomide, diethylbenzamide /272/. We shall also note, that studying of isomerization of HFPO is of practical interest as well, because on the basis of PFPrF a corresponding diacyl peroxide can be synthesized, which is an effective initiator of co-polymerization of fluorolefines /276/.
4.1. Isomerization of Hexafluoropropyleneoxide to Perfluoropropionylfluoride (PFPrF)
Preliminary tests of thoroughly dehydrated unmodified activated charcoal of AP-B grade displayed high catalytic activity in isomerization of HFPO to PF: thus, at 423 K contact period of 10 sec a full conversion of HFPO was seen.
For a quantitive estimation of activity of nucleophilic centers of carbon surface it was interesting to compare kinetic characteristics of isomerization of HFPO at activated charcoal and typical nucleophilic catalyst – alkali metals fluorides. In connection to this we have prepared and tested dehydrated cesium, potassium and sodium fluorides; those tests have revealed low effectiveness of individual fluorides of alkali metals, which was caused by their low activity and, the main thing, by mechanical decay in the course of process, that led to abrupt increase of catalyst layer resistance.
It is known, that dispersion of active component at a carrier in a number of cases allows significant increasing of effectiveness of catalytic impact, that is caused, mainly, by growth of active phase specific surface /277/; using of mechanically resistant cable provides high durability of applied catalyst in this case.
In this connection we have prepared and tested applied catalysts – cesium, potassium and sodium fluorides dispersed on the surface of melted calcium fluoride, which is of high mechanical resistance, and besides that, it is resistant/stable in fluorinating media. The important thing here is of course the fact, that this carrier is of electrophilic activity, providing for occurrence of side reaction – isomerization of HFPO to perfluoroacetone (PFA) /272, 278-284/.
Data on catalytic properties of applied alkali metals fluorides, and also unmodified, initial melted calcium fluoride are listed in Table 38, here as well you can see the results on catalytic properties of preliminary dehydrated activated charcoal.
Perfluoroacetylfluoride and tetrafluoroethylene are the main products of HFPO transformation at unmodified calcium fluoride; conversion degree at 483 K in this case amounted to as low as 10,7%. Modifying of calcium fluoride using alkali metals fluorides leads to sharp increase of isomerization rate; thus, applied cesium and potassium fluorides display their significant activity even at 410-430 K, at 470-480 K we observe virtually total transformation of HFPO, the yield of PFPrF at that reaches 99,6 %.
Preliminary dehydrated activated charcoal (AC) is of exclusively high initial activity in isomerization of HFPO.
Table 38. Catalytic Isomerization of Hexafluoropropyleneoxide (volume rate of substrate input – 250 hour-1)
|
# |
Catalyst-1 |
Tem-pera-ture, K |
HFPO Conversion Degree, % |
Products Yield, wt. % |
|||
|
Isomerization |
Destruction |
Oligomerization |
|||||
|
PFPrF |
PFA |
||||||
|
1 |
CaF2 |
483 |
10,7 |
24,2 |
<0,1 |
75,8 |
<0,1 |
|
2 |
Same |
573 |
98,2 |
2,6 |
<0,1 |
97,4 |
<0,1 |
|
3 |
NaF/CaF2 |
433 |
8,4 |
99,9 |
<0,1 |
0,1 |
<0,1 |
|
4 |
Same |
473 |
84,4 |
99,4 |
<0,1 |
0,6 |
<0,1 |
|
5 |
KF/CaF2 |
413 |
12,5 |
99,9 |
<0,1 |
0,1 |
<0,1 |
|
6 |
Same |
478 |
99,2 |
99,5 |
<0,1 |
0,5 |
<0,1 |
|
7 |
CsF/CaF2 |
413 |
18,7 |
99,9 |
<0,1 |
0,1 |
<0,1 |
|
8 |
Same |
473 |
99,2 |
99,6 |
<0,1 |
0,4 |
<0,1 |
|
9 |
AC2 |
373 |
97,8 |
93,7 |
2,0 |
0,3 |
4,0 |
|
10 |
Same |
473 |
99,5 |
95,0 |
3,4 |
0,8 |
0,8 |
1Amount of applied sodium, potassium and cesium fluorides was 6,6 mmole (per one) per 100 g of catalyst.
2Activated charcoal (AC) for decreasing of electrophilic activity of its ash part was preliminary treated using aqueous solution of potassium hydroxide (20 wt. %) at 373 K in 5 hours and flashed with water; listed data correspond to initial period of carbon’s activity (in 1 hour after start of experiment ).
It allows us carrying out total conversion of substrate even at 373 K.
It is typical, that modification of activated charcoal using cesium and potassium fluorides doesn’t lead as the tests proved to the increase of catalytic activity; at that, the stability of influence somewhat decreases.
Studying the kinetics of isomerization of HFPO using fresh activated charcoal (by the initial reaction rates), carbon after stabilization of its activity in time, and also alkali metals fluorides applied onto calcium fluoride showed, that in the field of initial concentrations of HFPO 6,7*10-4 – 2,1*10-3mole/l, temperature range of 383-573 K and conversion degrees up to 12% that reaction passes according to the first order.
Kinetic scheme of isomerization of HFPO can be presented in the following form:
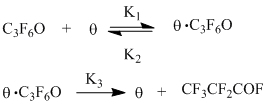
That scheme includes reversible adsorption of HFPO at free catalytic centres Θ (equation 1) and following isomerization with forming of (PFPrF) and regeneration of active centers (equation 2).
The reaction rate (W) according to presented scheme will be described by equations:
![]() (quasi-equilibrium
approximation)
(quasi-equilibrium
approximation)
![]() (quasi-stationary approximation)
(quasi-stationary approximation)
Where в = K1/K2 – adsorption coefficient (constant of adsorption equilibrium) of HFPO.
At small meanings of concentration of HFPO (вC<<1) in quasi-equilibrium approximation W=K3вC=Keffective C, that coincides with experimentally defined first order of reaction rate by concentration of HFPO.
Data on temperature dependencies of HFPO isomerization rates for studied catalysts are listed in Pic. 9; in Table 39 the values of corresponding pre-exponential mulripliers and activation energies are listed.
The energy of activation of HFPO isomerization at fresh activated charcoal in the temperature range of 443-543 K amounted to as low as 15,1 kJ/mole, that indicates a passing of reaction within the pore diffusion field /285/. At temperature range of 383-413 K the reaction transfers apparently into kinetic filed, about which a sharp increase of activation energy indicates, in this case it amounts to 50,2 kJ/mole. It is quite interesting, that the activation energy of isomerization at wasted activated charcoal (after stabilization of its activity in time) amounts to 56,5 kJ/mole in all studied range of temperatures and it is close to the meaning of reaction activation energy at fresh activated charcoal in kinetic field.
The energy of HFPO isomerization activation is naturally decreasing in the row of applied sodium, potassium and cesium fluorides (93,3; 81,6 and 55,6 kJ/mole, respectively), that indicates the increasing of activity of ion-fluorine with the growth of alkali metal cation radius and coincides with the results of numerous researches on studying the nucleophilic activity of ion fluorine /286,287/.
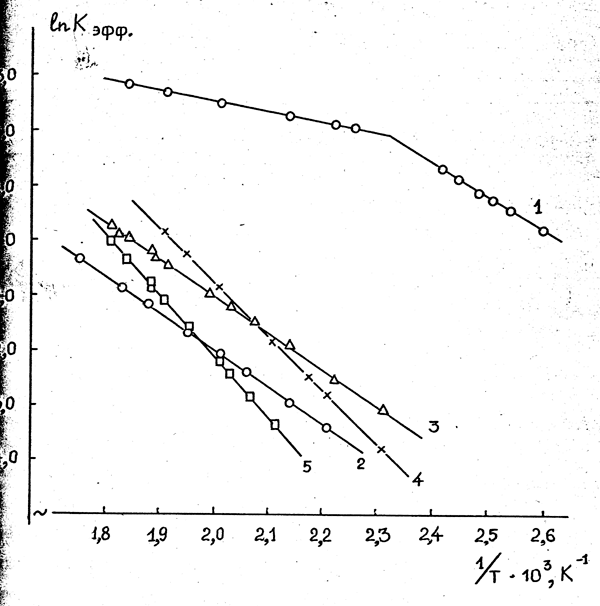
Picture 9.Temperature Dependencies (in Arrenius coordinates) of Constants of Isomerization Rates (Keffective) of Hexafluoropropionylfluoride at fresh (1) and wasted (2) activated carbon, and also at cesium (3), potassium (4) and sodium (5) fluorides applied onto calcium fluoride.
Table 39. Kinetic Characteristics of Catalytic Isomerization of Hexafluorinepropyleneoxide to Perfluoropropionylfluoride.
|
#. |
Catalyst |
Temperature Range, K |
lg Kо |
Еact., kJ/mole |
|
1 |
Activated Charcoal (Fresh) |
383-413 |
6,9±0,4 |
50,2±3,3 |
|
2 |
Same |
443-543 |
2,6±0,1 |
15,1±0,8 |
|
3 |
Activated Charcoal (Wasted) |
453-573 |
5,0±0,1 |
56,5±0,8 |
|
4 |
CsF/CaF2 |
433-553 |
5,3±0,1 |
55,6±0,8 |
|
5 |
KF/CaF2 |
433-523 |
8,2±0,1 |
81,6±0,8 |
|
6 |
NaF/CaF2 |
473-553 |
8,8±0,1 |
93,3±0,8 |
It is interesting, that activation energy of HFPO isomerization at activatedCharcoaland the one at applied cesium fluoride are rather close (50,2 and 55,6 kJ/mole, respectively), that indicates a high nucleophilic activity of catalytic centers of charcoal surfaces. This result provoked further works regarding studying of catalytic properties of activated charcoal in practically important reactions of fluoroorganic compounds passing according to nucleophilic type.
We shall point out, that according to the results of this part of work the initial data regarding projecting pilot plant obtaining perfluoropropionylfluoride by catalytic isomerization of hexafluoropropyleneoxide have been issued.
4.2. Selective Thermal Destruction of Carbonyldiflupride And Perfluorinated Polyperoxides’ Oligomers Over Perfluoropolyoxamethylenacetylfluorides
Low-molecular perfluoropolyoxamethylenacethylfluorides CF3O(CF2O)nCF2COF of medium molecular weight ~ 300 (RFAn), which is precious raw material to obtain new generation of thermal and frost aggression resistant fluororubbers of unique properties /287, 288/ are formed during thermal liquid-phase oxidation of HFP by oxygen in the medium of 1,1,2-trifluorotrichloroethane /289/; at that, the yield of RFAn is not that big and amounts to 1,8-2,0 mass. %. We have developed and introduced the obtaining technology of RFAn and HFPO by thermal liquid-phase oxidation of HFP using oxygen in the medium of 1,1,2-trifluorotrichloroethane with the additives of selective initiator– 1,2-dibrominetetrafluoroethane /290/, that allowed significant increasing of the rate of the process/291/, increasing (1,5 times) the yield of RFAn /292/ and HFPO – from 36-37 up to 48-49% /293/, and also decreasing the specific input of destructive oxidation (“burning”) of HFP /294/, which is going on forming by-products – carbonylfluoride and acetylfluoride.
Along with RFAn in the course of HFP oxidation the oligomers of carbonylfluoride with ending fluoroformate group СF3O(CF2O)nCOF (FFG) and perfluorinated polyperoxides (PFP) are formed, which are being accumulated in solvent and they also hamper the transportation and isolation of RFAn. That is causing the necessity of preliminary purification of RFAn solutions from FFG and PFP.
There is a report /295/ on the possibility of decomposition of FFG and PFP during their thermal processing in vacuum (470-500 K); at the same time the necessity of carrying out the process at lowered pressure will create additional difficulties during industrial implementation of such purification method.
In /296/ a two-stage purification method is developed, which includes solution contacting (at first stage) the catalyst of FFG decomposition – anhydrous potassium fluoride in a periodical mode and then – at second stage – thermal treatment using initiator of decomposition PFP – dimethyl ether of diethyleneglycole (diglime). Though, such a two-stage method allows reaching high degree of purification of RFAn from FFG and PFP, is rather complicated it is characterized by high energy and materials consumption, low productivity and waste – wasted potassium fluoride and diglime. At the same time listed in /296/ data point out an opportunity of intensification of decomposition of FFG by the catalysts of nucleophilic type. In connection to that we have studied the gas-phase destruction of FFG and PFP in the mixture with RFAn at preliminary dehydrated activated charcoal, which as it was mentioned before, is a promising catalyst of nucleophilic type. Carrying out the thermal destruction in gas phase at stable layer of firm catalyst has got its evitable technological advantages, particularly, it allows setting up an on-going process; at that, there is no need in additional stage of catalyst isolating from reaction products. We shall also notice, that besides high activity and stability in decomposition of both FFG and PFP the absence of thermal destruction of target products - RFAn at it is one of the main demands put to the catalyst.
Data on thermal destruction of FFG and PFP in the mixture with RFAn at preliminary dehydrated activated charcoal are listed in Table 40; here to compare you will find the results obtained in an empty reactor without catalyst and at typical catalysts of nucleophilic type – carefully and thoroughly dehydrated potassium and sodium fluorides.
Table 40. Thermal and Catalytic Decomposition of Perfluorinated Polyperoxides, Oligomers of Carbonyldifluoride and Perfluoropyoxamethylacetylfluorides.1
|
#. |
Catalyst |
Temperature, K |
Volume rate of Reagents’ Input, hour-1 |
Decomposition Degree, % |
||
|
PFP |
FFG |
RFAn |
||||
|
1 |
Without Catalyst |
473 |
3600 |
11,1 |
6,2 |
0,3 |
|
2 |
Same |
873 |
3600 |
99,0 |
99,0 |
9,9 |
|
3 |
-«- |
473 |
360 |
29,7 |
19,5 |
2,9 |
|
4 |
-«- |
723 |
360 |
99,2 |
99,0 |
12,9 |
|
5 |
NaF |
473 |
3600 |
14,7 |
27,9 |
15,1 |
|
6 |
KF |
473 |
3600 |
2,3 |
61,1 |
2,9 |
|
7 |
Activated Charcoal |
373 |
3600 |
85,3 |
96,4 |
0,8 |
|
8 |
То же |
373 |
360 |
99,5 |
99,0 |
1,8 |
|
9 |
-«- |
358 |
400 |
99,5 |
99,0 |
<0,1 |
|
10 |
-«- |
353 |
360 |
99,5 |
99,0 |
<0,1 |
1The results of experiments 9 and 10 are obtained under pilot conditions.
From the listed data it can be seen, that PFP, FFG and particularly RFAn are rather thermal resistant compounds. At temperature of 573 K and volume rate of input of 3600 hour-1 their decomposition degree (in an empty tube, without catalyst) amounted to as low as 18,4; 10,4 and 2,7%, and at 773 K – 98,2; 46,8 and 5,7% respectively.
Practically total non-catalytic decomposition of FFG and PFP (volume rate of reagents’ input - 3600 hour-1) is reached at only 873 K. Exclusively high thermal resistance of RFAn is drawing the attention, which degree of decomposition under the same conditions amounts to as low as 9,9 %.
The decreasing of volume rate of reagents’ input down to 360 hour-1allows lowering the temperature of total catalytic decomposition of FFG and PFP to 723 K; at that, RFAn destruction degree is 12,9 %.
A conducted study allowed quantitive estimating of comparative thermal stability of individual perfluoropolyoxamethylacetylfluorides with different number of carbon atoms (Pic. 10).
It can be seen. That thermal stability of RFAn significantly depends on the length of their chain. Thus, at 873 K and contact period of 1 sec (volume rate of reagents’ input of 3600 hour -1) perfluoropolyoxamethylenacetylfluorides with n = 1 are not decomposing ; the degree of decomposition of RFAn with n = 2, 4, 6 and 9, for example, is 6,0; 9,0; 12,6 and 19,0 %, respectively.
Data on relative thermal stability of PFP, FFG and RFAn (Table 40, experiments 1-4) point out the principal opportunity of thermal non-catalytic purification of perfluoropoluoxamethyleneacetylfluorides. The disadvantage of such method is a need to carry out the process at high temperature (720-870 K), that leads to increased power inputs for heating and cooling of reaction mixture. Besides that, under conditions providing total decomposition of PFPand FFG one can observe a significant destruction of target products (approximately 10%). That causes the purposefulness of selection of selective bifunctional catalyst allowing lowering the temperature of total decomposition of PFP and FFG.
Testing of potassium and sodium fluorides revealed the increase of gas-phase decomposition of FFG and PFP; at the same time these catalysts are not stable enough. Thus, the initial decomposition degree of PFP at potassium fluoride (volume rate of input - 3600 hour-1) at 373 K was equal to 13,8 %, while in six hours it amounted to 2,3 % even at 473 K. Besides that, potassium fluoride is being mechanically decomposed in the course of process, that leads to fractioning of sample’s granules and clogging of reactor. Catalyst decomposition can be caused by its reversible interaction with fluoroanhydrides forming alkooxides, that leads to alteration of phase composition.In case of FFG such alkoxides are decomposing isolating carbonyldifluoride and fine-dispersed potassium fluoride, which provokes reactor clogging.
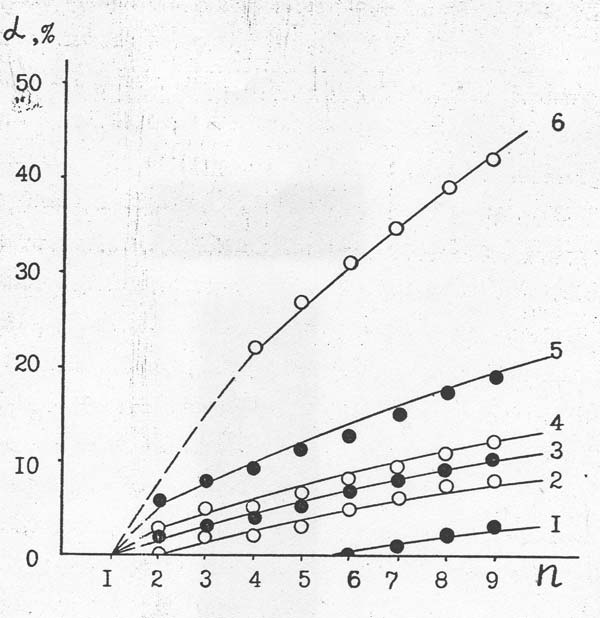
Picture 10. Dependency of Thermal Destruction (α) of Individual Perfluoropolyoxamethyleneacetylfluorides On The Length of Their Chain (n) At Volume rate Of Input Equal to 3600 hour-1And Temperature: 1 – 473 K; 2 – 573 K; 3 – 723 K; 4 – 773 K; 5 – 873 K и 6 – 923 K.
Samples obtained by applying potassium fluoride onto CaF2 and α-AI2O3 оare of significant mechanical durability and do not decompose in the course of operation; at the same time the activity of these contacts is not high (lower, than the one of individual potassium fluorides) and, besides that, it decreases in time.
Low stability of action can be caused by blocking of active centers by high-molecular fluoroanhydrides.
Preliminary dehydrated activated charcoal, as we see judging by data listed in Table 40 is of very high catalytic activity. At 323 K and volume rate of reagents input of 360 hour-1 (contact time for cold gas – 10 seconds) practically total decomposition of PFP and FFG is observed (98,4 and 94,8 %, respectively); decreasing of contact time results in decreasing of purification degree. Thus, at volume rate of input of 720 hour-1 (323 K) the purification degree from FFG and PFP is 84,2 and 92,8 %, and at 1440 hour-1 – 48,0 and 80,8 %, respectively. Decreasing temperature to 290-300 K at contact time also results in decreasing of purification degree.It follows from the data presented, that at industrial implementation of method the temperature and time must exceed 320 K and 10 seconds, respectively to reach the high degree of purification. It is typical, that contacting of perfluoropolyoxamethyleneacetylfluorides with activated charcoal even under relatively severe conditions (temperature 523 K, contact period of 10 sec) did not result in their destruction.
Thus, using catalyst – activated charcoal allows decreasing temperature of total purification of RFAn from PFP and FFG from 720-870 K (for non-catalytic process) to 320-370 K and escaping unwanted destruction of target products.
Concluding this part of the work we shall notice, that industrial tests confirmed the results of laboratory studies (Table 40, experiments 9, 10) and highlighted high stability of action of activated charcoal: processing of more than 3 tons of mixture per 1 kg of catalyst (exploitation for 2 years) did not result in decreasing of its activity – purification degree from FFG and PFP exceeded 99%.
Industrial implementation of method allowed setting up systematic processing of RFAn, in course of which obtaining of new types of thermal and frost resistant fluororubbers /287/ - SKF-260 (in accordance with Russian Space Shattle "Buran" programme) and SKF-Ftorkam had been carried out
4.3. Reactions of Perfluoroisobutylene with Anhydrous Hydrogen Fluoride, 1,1,5-trihydroperfluoropentanol-1 and Water
HFP synthesis technology – raw material for obtaining of fluoropolymers, fluororubbers and a whole number of other fluoroorganic products is based on pyrolysis of TFE. At pyrolysis of TFE highly toxic perfluoroisobutelene (PFIB, Maximum permissible concentration of PFIB in the air of working area is equal to 0,1 mg/m3) is formed along with HFP, octafluorocyclobutane (OFCB), as well as a number of other fluoroorganic products (perfluorobutenes, perfluoromethylcyclobutan and others); that’s why it is necessary to preliminary purify pyrolysis products* from PFIB to provide safety of their separation.
*During the rectificative separation of pyrolysis products the unreacted TFE and OFCB (they are being returned to the stage of pyrolysis) and bottom products, which are neutralized later, are being isolated, besides target product of HFP.
PFIB high activity (compare to TFE and HFP) in reactions with nucleophilic reagents /297-303/ is its characteristic feature; virtually all known methods of its selective isolation out of TFE pyrolysis products are based on that feature. Thus, initially, the purification of the products was carried out by liquid phase hydrolysis of PFIB to hexafluoroisobutyric acid by water in the mixture with acetone /304/; that method was characterized, however, by insufficient fullness of PFIB isolation, as well as fire and explosion hazard caused by acetone use. Now PFIB are hydrolized by aqueous solution of sodium hydroxide over catalyst of nucleophilic type – triethylamine /305/. That provides practically total selective elimination of PFIB and, besides that, it allowed setting isolation and recycle for pyrolysis of additional quantities of OFCB and perfluorobutenes (from bottom products), that led to decrease of specific consumption of TFE, and also to decreasing of environmental pollution by bottom products /306,307/. Total mineralization of PFIB, which is precious fluoroorganic raw material is a disadvantage of that method.Taking into account significant amounts of forming PFIB (130-140 kg per 1 ton of HFP), the search for ways allowing carrying out its utilization along with total isolation from pyrolysis products is essential.
The Timoshenko' Military Academy of Chemical Defense has developed in cooperation with us an utilization method of PFIB, which lies in contacting of TFE pyrolysis products with 1,1,5-trihydroperfluoropeptanole-1 at catalysis by potassium fluoride /185,186/; as a result of selective interaction of PFIB with this fluoroalcohol a forming of 2,5,5,9-tetrahydro-2-perfluoromethyl-4-oxaperfluorononane occurs /186/. Direct selective fluorination of this product allows obtaining effective dielectric liquid -2-perfluoromethyl-4-oxaperfluorononane.
Another way to utilize the PFIB may be based on its selective hydrofluorination by hydrogen fluoride forming monohydroperfluoroisobutane – effective working body for plasmochemical teratmnet of electronic schemes elements /297/.
Studying an opportunity of gas-phase catalytic hydrolysis of PFIB using water is promising; application of such method would allow significant simplification of technology of additional purification of bottom products.
It is characteristic, that all these reactions – interaction of PFIB and hydrogen fluoride, 1,1,5-trihydroperfluoropentanole-1 and water are the processes passing according to nucleophilic type; in this connection we have studied their catalysis by activated charcoal.
Interaction of Perfluoroisobutylene And Fluorohydric Acid.
Information on interaction of PFIB within the composition of products of TFE with hydrogen fluoride pyrolysis over dehydrated activated charcoal (AC) is presented in Table 41; here for you to compare the results obtained using empty reactor (without catalyst) over typical catalysts of nucleophilic type-thoroghly dried fluorides of cesium and potassium, applied onto surface of melted calcium fluoride are listed.
One can see from the listed data, that PFIB in the gas phase without catalyst doesn’t interact with fluorhycric acid (at temperature up to 570 K and volume rate of reagents input of 80 hour-1).
Though contacting of PFIB with hydrogen fluoride in the layer of applied fluorides of cesium and potassium (experiments 3, 4, Table 41) is accompanied with formation of some amounts of monohydroperfluoroisobutane it is characterized by low conversion degree of substrate (less then 15%).
Using activated charcoal results in sharp increase of PFIB and HF interaction rate (experiments 5-8, Table 41). Thus, at as low as 323 K and volume rate of reagents’ input equal to 750 hour-1conversion degree of PFIB amounts to 68%; increasing of temperature to 373-423 K is accompanied by total conversion of PFIB (conversion degree above 99%). It is characteristic, that over activated charcoal the hydrogen fluoride reacts in a rather selective way – the content of of monohydroperfluoropropane – product of hydrafluorination of HFP in reaction gases even at 423 K doesn’t exceed 0,1 of volume %.
High stability of catalytic action of activated charcoal must be noted – its operation during 400 hours (423 K, volume rate of reagents’ input – 80 hour-1, mole ratio of HF: PFIB – 1,5) doesn’t result in decreasing of PFIB conversion degree, which under these conditions doesn’t exceed 99%.
Interaction of Perfluoroisobutylene And 1,1,5-trihydroperfluoropentanole-1.
Contacting of TFE pyrolysis products with 1,15-trihydroperfluoropentanole-1 over activated charcoal at 473 K (experiment 10, Table 41) also results in practically total selective conversion of PFIB; at that, catalyst is characterized by high stability of action. Decreasing temperature down to 373 K and below is accompanied by decreasing of completeness of PFIB binding by fluoroalcohol (to 85%); in this case the activity of catalyst is significantly falling in time, that can be caused by irreversible adsorption (at these temperatures) of 1,1,5-trihydroperfluoropentanle-1 or its high-molecular adducts with PFIB at catalytic centers of carbonic surface.
Interaction of Perfluoroisobutylene And Water.
As it follows from data on interaction of PFIB with waterlisted in Table 41 (experiments 11-14), without catalyst this reaction practically doesn’t occur (to 473 K). Application of applied potassium fluoride allows significant increasing of PFIB hydrolysis rate – at 423 K its conversion degree amounted to 42%. Catalytic activity of carbon though is significantly higher; under the same conditions the PFIB conversion degree exceeds 99%.
The formation of significant amounts of 2-hydroperfluoropropylene (see Table 41), and also 2,2-dihdroperfluoropropane (its concentration in reaction gases in experiments 13, 14 amounted to 0,84 and 0,43volume % or 25 and 13 mole %, respectively, of PFIB having entered the reaction) is a characteristic feature of catalytic gas-phase interaction of PFIB and water.
Chemism of formation of these admixtures can be described using the following scheme:
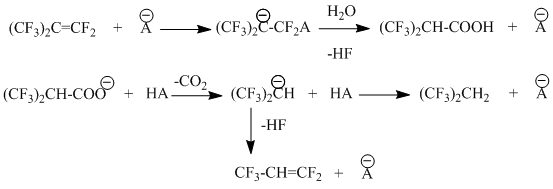
This scheme includes a stage of nucleophilic attack of PFIB during interaction with catalytic centre
![]() , hydrolysis of PFIB
activated in such way forming hexafluoroisobutyric acid or its anion, decarboxylation of this anion
forming carbanion
, hydrolysis of PFIB
activated in such way forming hexafluoroisobutyric acid or its anion, decarboxylation of this anion
forming carbanion ![]() ,
which either attaches proton forming 2,2-dihydroperfluoropropane or transforms into 2-hydroperfluoropropylene
eliminating fluoride-ion.
,
which either attaches proton forming 2,2-dihydroperfluoropropane or transforms into 2-hydroperfluoropropylene
eliminating fluoride-ion.
Forming of hexafluoro-iso-butyric acid carbanion is rather possible, if we take into account the presence of nucleophilic centers on carbon’s surface, which are promoting its dissociation; besides that, the appearance of decarboxylation products- C3F6H2and C3F5H is observed at relatively low temperature range (300-320 K), e.g. under conditions, which are not typical for decarboxylation of hexafluoroisobutyric acid, while its anion is of significantly lower resistance.
We shall notice, that pentafluoropropylene forming during PFIB hydrolysis hampers the quality of target product – HFP. Taking into account the difficulty of rectification isolation of pentafluoropropylene from HFP it looks reasonable to separate TFE pyrolysis products in advance into two fractions: light, containg unreacted TFE, HFP and part of OFCB and heavy one, containing part of OFCB, PFIB and other high-boiling pyrolysis products. At that only heavy fraction, which doesn’t contain HFP, that excludes the possibilty of its contamination with pentafluoropropylene forming at PFIB hydrolysis should undergo purification from PFIB.
On the other hand, additional purification of bottom products from unreacted PFIB is one of the stages of HFP production, as it has been noticed before. After purification these products are not utilized; carrying out of this process by thermal hydrolysis at activated carbon would allow significant simplification of additional purification technology.
The tests proved, that contacting of bottom products with water (water content was 10-15% of their weight) at activated charcoal at 420-470 K and volume rate of reagents input of 180-300 hour-1 is characterized by total purification from PFIB.
According to the results of this part of the work the initial data on constructing/ projecting a waste-free purification technology of TFE pyrolysis products from PFIB (using hydrofluorination) and of bottom products in HFP production (using hydrolysis).
4.4. Thermal Destruction of ω–chloroperfluoroalkylfluorosulphates
The Timoshenko' Military Academy of Chemical Defense in association with us have developed the technology of utilization of α-hydro-ω–chloroperfluoroalkanes H(CF2)nCl – TFE production waste /308/. ω-Chloroperfluoroalkylfluorosulphates /187,188,309-311/ are formed as intermediate products by this technology, they are being processed into corresponding fluoroanhydrides of ω-chloroperfluorocarboxylic acids /189/ with further obtaining of effective medical preparations on their basis, flame extinguishing agents, herbicides and fluoroemulsifiers /312-315/. In connection to that the selection of effective catalysts of selective thermal destruction of chlorinealkylfluorosulphates to corresponding fluoroanhydrides is important:
Cl(CF2)nOSO2F → Cl(CF2)n-1COF+SO2F2
According to /189,316/ typical catalysts of nucleophilic type – alkali metals fluorides further decomposition of alkylfluorosulphates. In connection to that we have studied the opportunity of ω-chlorperfluoroalkylsulphates thermal destruction catalysis, particularly ω- chloroperfluorooctylfluorosulphate by preliminary dehydrated activated charcoal; data is presented in Table 42.Here for you to compare the results on decomposition of ω-chloroperfluorooctylfluorosulphate in the layer of inert nozzle – melted calcium fluoride are listed.
Table 42. Catalytic thermal destruction of ω–chloroperfluoroctylfluorosulphate (volume rate of substrate input - 360 hour-1)
|
Catalyst |
Conversion Degree of Cl(CF2)8OSO2F,%, at Temperature, K |
|||
|
473 |
523 |
573 |
673 |
|
|
CaF2 |
0,5 |
0,5 |
1,7 |
9,1 |
|
Activated Charcoal grade AP-B |
6,2 |
21,5 |
52,1 |
98,2 |
One can seefrom the listed data, that activated charcoal abruptly increases the rate of thermal destruction of ω-хлорперфтороктилфторсульфата; ω-chloroperfluorooctanoilfluoride is virtually only one organic product of this process.
4.5. Hydrolysis of 1,1,1-trifluorotrichloroethane in the Mixture with 1,1,2-trifluorotrichloroethane
1,1,1-Trifluorotrichloroethane is an undesirable admixture in 1,1,2-trifluorotrichloroethane; its presence, in particular, decreases the stability of mixture chladone-113 isomers, and also it is accompanied by pollution of trifluorochloroethylene obtained on the basis of 1,1,2-trifluorotrichloroethane by hard to separate admixture – 1,1-difluoro-chloroethylene, which worsens the quality of this fluorochlorolefine. In connection to that the search for purification ways of 1,1,3-trifluorotrichloroethane from 1,1,1-trifluorotrichloroethaneis quite actual. These isomers of R-113 share the same boiling point and they do not separate from each other by rectification.
In the present part of work the results on selective nucleophilic hydrolysis of small amounts of 1,1,1-trifluorotrichloroethane (0,8 wt. %) in the mixture with 1,1,2-trifluorotrichloroethane at activated charcoal (Table 43) are listed; here for you to compare the data on hydrolysis of these isomers over potassium fluoride (10 wt. %) applied onto γ-Al2O3are listed.
Table 43. Catalytic Hydrolysis of 1,1,1-trifluorotrichloroethane in the mixture with 1,1,2-trifluorotrichloroethane (temperature 473 K, mole ratio water : trifluorotrichloroethane isomers sum = 2).
|
# |
Catalyst |
Volume rate of Reagents Input, hour-1 |
Conversion Degree, % |
|
|
CF3-CCl3 |
CF2Cl-CFCl2 |
|||
|
1 |
γ-Al2O3 |
360 |
<0,1 |
<0,1 |
|
2 |
KF/ γ-Al2O3 |
360 |
6,2 |
1,4 |
|
3 |
Activated Charcoal grade AP-B |
360 |
34,1 |
1,4 |
|
4 |
Same |
180 |
53,0 |
2,8 |
|
5 |
-//- |
150 |
62,0 |
3,5 |
|
6 |
-//- |
120 |
70,1 |
4,3 |
|
7 |
-//- |
90 |
82,7 |
5,6 |
One can see from the listed data, that rate and selectivity of hydrolysis at activated charcoal is significantly higher, than over applied potassium fluoride; using of activated charcoal allows carrying out deep selective hydrolysis even of small amounts of 1,1,1-trifluorotrichloroethane under quite mild conditions (temperature 473 K, volume rate of reagents input – 90 hour -1).
4.6. The Origin of activated charcoal’s Catalytic Influence
Revealing of origin of activated charcoals catalytic influence is a rather complicated task, that is caused by exclusively rich chemistry of their surface: besides non-organicadmixtures (ash of activated charcoals contains /317/ aluminium, silicon, iron, magnesium, calcium and potassium) they usually find hydrogen, oxygen, sulphur and nitrogen /318/ in carbonized products. All of that hampers referring one or another catalytic property to any definite group of centers. It is known, that surface of activated charcoals contains significant amounts of chemosorbated oxygen. Detailed studies /318/ allowed referring practically all of the oxygen defined by elementary analysis to well -known functional groups: carboxylic, hydroxylic (phenol), carbonyl and lactone.Nucleophilic pieces of such surface can be responsible for catalytic influence of activated charcoal in studied reactions.
In connection to that we have conducted experiments using mixture of benzoic acid, phenol and succinic anhydride; functional groups of these compoundsshape oxygen containing fragments of carbonic surface. The contact of such mixture with PFP and FFG at 298 K, as it has been stated in experimental way, doesn’t lead to their destruction. Here it should be noted, that hydroxyl, carbonyl, carboxylic and lactone groups of carbonic surface can possess somewhat another properties than the same groups in the composition of used model mixture. It doesn’t allow making a definite conclusion on participation of oxygen containing fragments on the surface of activated charcoal in nucleophilic reactions of fluororganic compounds.
The surface of activated charcoal can contain free-radical centers as well, forming at the stage of activating, which is accompanied by burning out of part of crystallites carbon and decomposition of hexahonal rings with apprearing of side chains of carbon atoms. Such radicals according to /318/ possess nucleophilic properties and can accelerate studied reactions. Chemisorption of water at radical carbon fragments results in formation of ion-exchange hydroxylic groups of main character /318/. Their replacement for fluorine at contact with chemically responsive fluoroorganic compounds can result in appearance of catalytic system with mobile ion-fluorine of nucleophilic properties; at that, activated charcoal plays a role of macro-cation and, besides that, promotes the increasing of substrates’ concentration (due to its high adsorption ability) around active centers.
In conclusion we shall notice, that studying of a wide range of thermal gas-phase reactions of polyfluorinated organic reactions of polyfluorinated organic compounds proved, that preliminary dehydrated activated charcoal is an effective universal catalyst of nucleophilic type and can be used even in unmodified form as a catalyst of hexafluorinepropyleneoxide isomerization, thermal destruction of oligomers of carbonyldifluoride and perfluorinated polyperoxides, hydrofluorination and hydrolysis of perfluoroisobutylene, as a well as its iteraction with 1,1,5-trihydroperfluoropentanole-1, decomposition of ω-chloroperfluoroalkylfluorosulphates and selective hydrolysis of 1,1,1-trifluorotrichloroethane in the mixture with 1,1,2-trifluorotrichloroethane.
The availability and simplicity of the use of this catalyst undoubtedly result in widening of the fields of its practical application.
5. CONCLUSION
1. Based on theoretical generalization of results of our study of gas-phase interaction of a wide range of unsaturated and hydrogen containing fluororganic substrates with elemental fluorine we have created new perspective scientific direction – the direct selective catalytic fluorination of polyfluorinated organic compounds.
- We have developed scientific principles of selection of effective aggression resistant catalysts of direct gas-phase fluorination of organic compounds and for the first time the opportunity of sharp increase of selectivity and fluorination rate using undiluted fluorine at directed alteration of nature of catalytic composition has been demonstrated.
- We have developed an effective universal all-purpose catalytic composition - NiF2 /α-AI2O3and using it, using the example of 3-perfluoroisopryl-2-perfluoromethylperfluoropentene-2 and 3-perfluoroisopropyl-4-perfluoromethylperfluoropentene-2 – the isomers of perfluorononene, isomers of 2-perfluoromethylperfluoropentene-3, isomers of perfluorobutene, 2,5,5,9-tetrahydro-2-perfluoromethyl-4-oxaperfluorononane, α-hydro-ω-chloroperfluoroalkanes H(CF2)nCl (n=2÷8), as well as polyfluorinated admixtures within the composition of fluorocarbonic lubricants, perfluorodecaline, perfluorodimethylcyclohexane and other fluorocarbonic liquids a selective gas-phase fluorination of polyfluorinted organic compounds has been carried out with quantitive yield of corresponding products of selective fluorination important in practical terms.
2. Based on systematic research of a wide range of thermal gas-phase transformations of polyfluorinated organic compounds it has been stated, that α-AI2O3 is a universal all-purpose, aggression resistant carrier for effective catalysts of thermal dechlorination of fluorochlorocarbons using hydrogen and fluorolefines oxidation using oxygen. Based on α-Al2O3:
- we have created stable catalysts of dechlorination using hydrogen of 1,2-dichlorohexafluoropropane to hexafluoropropylene (Ni/α-AI2O3), 1.2-difluorotrichloroethane to 1,2-difluorofichloroethylene (α-AI2O3; Ni/α-AI2O3), 1,1,2-trifluorotrichloroethane to trifluorochloroethylene (Cu,BaO/α-AI2O3), allowing significant increasing of rate as well as selectivity of these processes (from 70-80 up to 90-95% and above); kinectics and hydrogen fluorochlorocarbons dechlorination mechanism have been studied;
- an effective low-temperature catalyst of oxygen tetrafluoroethylene oxidation has been developed (cobalt oxide /α-AI2O3) with pointed obtaining of carbonyl fluoride.
3. As a result of studying of kinetic patterns/mechanisms of thermal gas-phase reactions of polyfluorinated organic compounds it is stated, that dehydrated activated charcoal is an effective aggression resistant catalyst of nucleophilic type and it can be used even in an unmodified form, particularly, for the catalysis of hexafluoropropylenoxide isomerization, thermal destruction of carbonyl fluoride and perfluorinated polyperoxides oligomers, hydrofluorination and hydrolysis of perfluoroisobutelene, as well as its interaction with 1,1,5-trihydroperfluoropenthanol-1, decomposition of ω-chloroperfluoroalkylfluorosulphates and selective hydrolysis of 1,1,1-trifluorotrichloroethane in the mixture with 1,1,2-trifluorotrichloroethane.
4. Based on the developed aggression resistant catalysts of thermal gas-phase transformations of polyfluorinated organic compounds the following technologies are introduced into production:
- technology of perfluorodimethylperfluorocyclohexane – an effective dielectric liquid for aviation technics;
- technology of fine purification of main component of developed artificial blood substituent – perfluorodecaline;
- perfluoropolyoxamethylenacetylfluorides purification technology that allowed total dump eliminating of those toxic fluoroanhydrides and obtaining of new generation of thermal and frost resistant fluorocaoutchoucs of unique properties based on them.
The following technologies have been introduced at a pilot scale:
- waste-free technology of fine purification of fluorocarbon liquids and lubricants.
- regeneration technology of waste fluorocarbonic lubricants and liquids;
- technology of new effective dielectric liquid - 2-perfluoromethyl – 4-oxaperfluorononane.
The initial data for design project have been issued. Among them there are the data for projecting of:
- production of pentafluorochloroethane (chladone-115, R-115), with elimination of dumping and utilization of octafluorocyclobutane – a waste of tetrafluoroethylene production;
- trifluoromethylhypofluoride technology – the reagent and initiator for the synthesis of a whole range of new practically important oxygen containing fluororganic compounds;
- hexafluoropropylene technology allowing eliminating of dumping and correspondingly, utilizing 1,2-dichlorohexafluoropropane – waste in the production of tetrafluoroethylene;
- technology of 1,2-difluorodichloroethylene, which excludes the formation and dumping of toxic zinc chloride;
- technology of carbonyl fluoride – a basis for production of a whole range of oxygen containing fluororganic compounds;
- waste-free tetrafluoroethylene pyrolysis products purification technology from perfluoroisobutylene (by hydrofluorination) and purification technology of bottom products in the production of hexafluoropropylene (by hydrolysis);
- pilot plant for selective isomerization of hexafluoropropylenoxide to perfluoropropionylfluoride – a basis for production of effective initiator of fluoroolefines co-polymerization.
References
2. Tedder D.M. Ftorirovanie organicheskih soedinenij ehlementarnym ftorom. V kn."Uspekhi khimii ftora", t. I-II -M.-L. : Khimiya, 1964, p. 380-423.
3. Orkin V.L., Chaikin A.M. Opredelenie konstant skorosti obrazovaniya atomov v reakciyah molekulyarnogo ftora s okis'yu azota, ehtilenom i tetraftorehtilenom. Kinetika i kataliz, 1982, t. 23, v. Z, p. 529-533.
4. Anson P.O., Fredricks P.S., Tedder J.M. Free-radical substitutionin aliphatic compounds. Part 1. Halogenation of n-butahe and isobutane in the gas phase. J.Chem.Soc, 1959, March, p.918-922.
5. Fredricks P.S., Tedder. J.M. Free-radical substitution in aliphatic compounds. Part 11. Halogenation of the n-butylhalides. -J.Chem.Soc, 1960, Jan., p. 144-150.
6. Purington S.T., Kagen B.S., Patric T.B. The application of elemental fluorine in organic synthesis. Chem.Rev., 1986, № 86, p.997-1018.
7. Aikman R.E., Lagow E.J. Syhthesis of tetra-cis-(perfluorocyclohexyl)-methane and bis-(perfluorocyclohexyl)-methane by direct fluorination. -J.Org.Chem., 1982, v.47, № 14, p.2789-2790.
8. Eremenko L.T., Oreshko G.V. Ftorirovanie ehlementarnym ftorom labil'nyh polinitrosoedinenij – adduktov reakcii Mihaehlya. -Izv. AN SSSR, Ser. khim, 1969, № 2, p.479.
9. Eremenko L.T., Nacibullin F.Ya., Borovinskaya J.P., Karpova N.D. Sintez perftornitroehtanov. -Izv. AN SSSR. Ser. khim., 1968, № 2, p.429-430.
10. Patent US 4523039. Sposob polucheniya perforirovannyh prostyh ehfirov. /Lagov R.J., Gerhart J.E. -Publ. 11.06.85, RZh Khimiya, 1986, 7N36.
11. Patent US 3242218. Sposob polucheniya ftoruglerodnyh poliehfirov. /Miller V.T. -Publ. 22.03.66, RZh Khimiya, 1967, 12C232.
12. Des Martean D.D, Fluoroperoxytrifluoromethane CF300F. Preparation from trifluoromethyl hydroperoxide and fluorine in the presence of cesium fluoride. -Inorg Chem., 1972, v.11, № 1, p.193-195.
13. Merritt R.F., Johnson F.A. Direct fluorination. Addition of fluorine to indenes and.acenaphthylenes, -J.Org.Chem., 1966, v.31. № 6, p.1859-1863.
14. Merritt R.F, Johnson F.A. Direct fluorination of steroidal olefines to cis-vicinal difluorides. –J.Am.Chem.Soc., 1966, v.88, № 8, p.1822-1823.
15. Patent US 3487093. Ftorirovannye olefiny. Merrit R.F. –Publ. 30.12.69, RZH Khimiya, 1970, 23N29.
16. Grakauskas V. Direct liquid phase fluorination of halogenated aromatic compounds. -J.Org.Chem., 1969, v.34, № 10, p.2835-39.
17. Brooke G.M., Chambers B.D., Heyes J., Musgrave W.K.R. Direct preparation and some reactions ofсhlorofluorobenzenes. -J.Chem.Soc., 1964, Febr., p.729-735.
18. Merritt R.F, The polar addition of molecular fluorine to acetylenes. –J.Org.Chem., 1967, v.32, № 12, p.4124-4126.
19. Sheppard U., Sharts K. Organicheskaya Khimiya ftora. -
M.: Mir, 1972, p. 52, 89, 112.
20. Miller V., Ehrenfeld J, Felan J., Prober M., Rid Sh, Ftorirovanie polnost'yu galoidirovannyh olefinov. «Khimiya ftora», № 2, -M.: IL, 1950, p. 228-240.
21. Miller W.T. My early days in fluorine chemistry. –J.Fluor.Chem., 1981, v.18, p.305-321.
22. Miller W.T., Stoffer J.O., Fuller G., Currie A.C. The mechanism of fluorination. IV. The effect of temperature and of fluorine concentration on reaction. A new fluorination apparatus. –
J.Am.Chem.Soc., 1964, v.86, № 1, p.51–56.
23. Isikava Nobuo, Kitacumeh Tomoya. Novye metody ftorirovaniya aromaticheskih soedinenij. –Yukki gosehj kachaku kyokajsi., 1976, t.34, № 3, p.173-178, RZh Khimiya, 1976, 23ZH360.
24. Patent JP 55-18695. Sposob ftorirovaniya./ Maruo Kehjiti, Misaki Susumu. –Publ. 21.05.80, RZh Khimiya, 1981, 5N122.
25. Sirip L.A., Lagow R.J. Direct fluorination of 2,2,4,4-tetramethyl pentane. Sterically protected residual protons. –J.Org.Chem., 1977, v.42, № 21, p.3437-3438.
26. Kowanko N., Branthaver J.F., Sugihara J.M. Direct liquid-phase fluorination of petroleus. –Fuel, 1978, v.57, № 12, p.769-775.
27. Patent US 40004996. Ftorirovanie organicheskikh soedinenij./ Kollonic J. –Publ. 25.01.77, RZh Khimiya, 1977, 23N57.
28. Patent GB 1077065. Ftorirovanie nitrosoedinenij./ Grakauskas V., Hemel E.E. –Publ. 26.07.67, RZh Khimiya, 1975, 16N84.
29. Merritt R.F. Direct fluorination of 1,1-diphenylethylene. –J.Org.Chem., 1966, v.31, № 11, p.3871-3873.
30. Merritt R.F. The polar fluorination of propenylbenzene. –J.Am.Chem.Soc., 1967, v.89, № 3, p.609-612.
31. Merritt R.F., Johnson F.A. Low-temperature fluorination of Schiff bases. -J.Org.Chem., 1967, v.32, № 2, p.416-419.
32. Misaki S. Direct fluorination of phenol and cresols. –
J.Fluor.Chem., 1981, v.17, № 2, p.159-171.
33. Maxwell A.F., Detoro F.E., Bigelow L.A. The action of elementary fluorine upon organic compounds. XXIII. The jet fluorination of certain aliphatic hydrocarbons as oriented and controlled by operation conditions. -J.Am.Chem.Soc., 1960, v.82, № 22, p.5827-5830.
34. Attaway J.A., Groth R.H., Bigelow L.A. The action of elementary fluorine upon organic compounds. XXIII. The fluorination of some amides, nitriles and of methylthiocyanate. -J.Am.Chem.Soc., 1959, v.81, № 14, p.3599-3603.
35. Robson P., Mc Longhlin V.C.R., Hynes J.B., Bigelow L.A. The action of elementary fluorine upon organic compounds. XXIV. The jet fluorination of hydrogen cyanide, cyanogen, methylamine and ethylenediamine. Pyrolysis and fluorinolysis of selected products. -J.Am.Chem.Soc., 1961, v.83, № 24, p.5010-5015.
36. Boffenberg K. Substitution von benzol durch elementares Fluor in der Gasphase. –Chem.Ztg.,1972, v.96, № 2, p.84-92.
37. Patent JP 58-41829. Poluchenie oktaftorpropana./ Fukui Siro, Joneda Hadzime. –Publ. 11.03.83, RZh Khimiya, 1984, 5N11.
38. Hayes L.J., Dixon D.D. Direct fluorination of polyester and related compounds. –J.Fluor.Chem., 1977, v.10, № 1, p.1-16.
39. Gerhardt G.E., Lagow R.J. Synthesis of perfluoropolyethers by direct fluorination: a novel preparation for perfluoro (polypropylene oxide) ethers and perfluoro (polymethylene oxide) ethers. –J.Chem.Soc., 1981, part 1, № 5, p.1321-1328.
40. Patent US 4113772. Metod polucheniya oligomerov perftorehfirov s koncevymi karboksil'nymi gruppami. / Lagov R.J. –Publ. 12.09.78, RZh Khimiya, 1979, 15N14.
41. Gerhardt G.E., Lagow R.J. Synthesis of perfluoropoly (ethylene glycol) ethers by direct fluorination. –J.Org.Chem., 1978, v.43, № 23, p.4505-4509.
42. Adcock J.L., Znoue Shoji, Lagow R.J. Simultaneous fluorination and functionalization of hydrocarbon polymers. –J.Amer.Chem., 1978, v.100, № 6, p.1948-1950.
43. Lagow R.J., Margrave J.L. The controlled reaction of hydrocarbopolymers with elemental fluorine. –J.Polym.Sci.: Polym.Lett.Ed., 1974, v.12, № 4, p.177-184.
44. Patent US 3775489. Process for fluorination of aromatic and polynuclear hydrocarbon compounds and fluorocarbons produced thereby. Margrave J.L., Lagow R.J. –Publ. 27.11.73.
45. Lagow R.J., Maraschin N.J. Direct fluorination of cyclic and bicyclic hydrocarbons. –7th Int.Symp.Fluorine Chem., Santa Cruz, Calif., 1973, s.l., s.a., p.1-25.
46. Gerhardt G.E., Dumitru E.T., Lagow R.J. Synthesis of hightbranched perfluoroethers by direct fluorination, promising new materials based on the hexafluoroacetone –ethylene copolymer. –J.Polym.Sci., 1980, v.18, № 1, p.157-169.
47. Robertson G., Liu E.K.S., Lagow R.J. Synthesis of perfluoroadamantane compounds by direct fluorination. –J.Org.Chem., 1978, v.43, № 26, p.4981-4983.
48. Lagow R.J. Large scale synthesis of organofluorine compounds using elemental fluorine; a third Simons cell. –Int.Symp. “Centenary of the discovery of fluorine”, Abstr., Paris, 1986, p.2.
49. Adcock J.L., Lagow R.J. The synthesis of the fluorinated ethers “perfluoroglyme” and “perfluorodiglyme” by direct fluorination. –J.Org.Chem., 1973, v.38, № 20, p.3617-3618.
50. Koshar R.J., Hausted D.R., Meiklejohn R.A. Organic fluoronitrogens. V. Bis(difluoroamino)difluoromethane. –
J.Org.Chem., 1966, v.31, № 12, p.4232-4234.
51. Koshar R.J., Hausted D.R., Wright C.D. Organic fluoronitrogens. VII. Tris(difluoroamino)fluoromethane and related compounds. –J.Org.Chem., 1967, v.32, № 12, p.3859-3864.
52. Patent US 3981783. Process ehlektrohimicheskogo ftorirovaniya s dopolnitel'noj podachej vodoroda i uvelicheniem vyhoda po toku. / Chailds V.V. –Publ.21.09.76, RZh Khimiya, 1977, 14L237.
53. Patent DE 2106870. Sposob ehlektrohimicheskogo ftorirovaniya organicheskih soedinenij./Voss P., Niderprum H., Kaul G., Trepp R., -Publ. 24.02.77, RZh Khimiya, 1977, 24N16.
54. Patent DE 2302132, 1976. Sposob polucheniya razvetvlyonnyh perftoralkanov. /Benninger S. -Publ. 23.12.76, RZh Khimiya, 1977, 24L202.
55. Patent US 4035250. Sposob polucheniya perftorgeptana. /Valters H.S., Chailds V.V. -Opubl.12.07.77, RZh Khimiya, 1978, 6N22.
56. Patent JP 53-18488. Sposob polucheniya perftorcikloalkanov. /Sato Dajsuke, Yamamuti Koiti, Murasima Ryoshiro. -Opubl. 15.06.78, RZh Khimiya, 1979, 9N121.
57. Patent US 3662009. Poluchenie nenasyshchennyh ftorsoderzhashchih soedinenij. /Hadchinson V.M. -Publ. 09.05.72, RZh Khimiya, 1973, 9N12.
58. Goldshtejn B.V., Serushkin I.L., Nikonorova N.I. O roli ftorida nikelya v reakciyah ehlektrohimicheskih soedinenij. –Tr. GIPKh, № 39, p. 1, inv. № T-1863., L., 1975, p.43-47.
59. Fauler R.D., Berford N.B., Gamilton J.M., Sweet R.J., Weber K.E., Kasper J.S. Laitain N. Sintez ftoruglerodov. «Himiya ftora», v. № 1 –M.: IL, 1950, p.91-113.
60. Benner R.J., Benning A.F., Downing F.B., Irvin S.F., Jonson K.S., Linch A.L., Parmeli H.M., Wirt W.W. Ftoruglerody, poluchennye ftorirovaniem uglevodorodov trekhftoristym kobal'tom. «Himiya ftora», v. № 1 –M.: IL, 1950, p.114-128.
61. Fauler R.D., Anderson H.S., Gamilton J.N., Berford N.B., Spagetti A., Biterdih S.B., Laitain N. Ftoridy metallov, primenyaemye v sinteze ftoruglerodov. V kn. «Himiya ftora», sb. № 1 –M.:IL, 1950, s.143-153.
62. Patent JP 60-81134. Poluchenie oktaftorpropana. /Katamura Koiti, Kagehyama Yutaka, Nakayama Hidehtosi. –Publ. 09.05.85, RZh Khimiya, 1986, 12N25.
63. Patent JP 60-10933. Poluchenie geksaftorehtana./ Katamura Koiti, Kagehyama Yutaka, Nakayama Hidehtosi. –Publ. 15.06.85, RZh Khimiya, 1986, 11N21.
64. Pak V., Peka J., Cermak V., Sykora F., Petrzila V. Poloprovozni zarizeni pro vyrobu perfluororganickych latek.
–Chem. Prum., 1978, v.28, № 9, p.467-470.
65. Patent FR 2381732. Usovershenstvovannyj sposob perftorirovaniya ciklicheskih uglevodorodov. / Moore R.E. –
Publ. 27.10.78, Izobreteniya v SSSR i za rubezhom, 1979, v.55, № 5, p.67.
66. Patent US 4143079. Sposob polucheniya perftor-1-metil-4-izopropilciklogeksana iz pinena. / Moore R.E. –Publ. 06.03.79, Izobreteniya v SSSR i za rubezhom, 1979, v.55, № 21, p.20.
67. Patent GB 1597914. Sposob perftorirovaniya ciklicheskih uglevodorodov. / Santehch inkorp. –Publ. 16.09.81, Izobreteniya v SSSR i za rubezhom, 1982, v.57, № 12, p.64.
68. Burdon J. The exhaustive fluorination of aliphatic compounds.
–Int. Symp. “Cent. ...”, Paris, 1936, p.9.
69. Moore R. E., Driscoll G. Perfluorination of bicyclic and tricyclic hydrocarbons. –IV-th Winter Fluorine Conf., Abstr, Daytona Bearen, 1979, p.8.
70. Asovich V.S., Prokudin I.P. Osobennosti ftorirovaniya organicheskih veshchestv chetyrekhftoristym ceriem. –Tr. GIPH, № 40, inv. № T-2117, L., 1976, p.25-31.
71. Isikava N., Kobayasi Yo. Ftor. Himiya i primenenie. –
M.: Mir, 1982, p.91.
72. Keidi J.H., Grosse A.V., Barber E.J., Berger L.L., Sheldon Z.D., Poluchenie ftoruglerodov kataliticheskim ftorirovaniem uglevodorodov. V kn. «Himiya ftora», v. № 1 –M.: IL., 1950, p.129-135.
73. Bigelou L.A. Dejstvie ehlementarnogo ftora na organicheskie soedineniya. «Ftor i ego soedineniya (pod red. J. Saimonsa), t.1 –M.: IL., 1953, p.314-335.
74. Musgrave W. K. R., Smith F. Organic Fluorides. PartІІ. The effect of metals on the fluorunationof hydrocarbons. –J. Chem. Soc., 1949, p.3026-3028.
75. Brik T. J. Ftoruglerody; ih svojstva i proizvodstvo vo vremya vojny. V kn. «Ftor i ego soedineniya» (pod red. Saimonsa J.), t.І –M.: IL, 1953, p.355-388.
76. Maraschin N. J., Catsikis B. D., Davis L. H., Larvinen J., Lagov R. J. Synthesis of structurally unusual fluorocarbons by direct fluorocarbon. –J.Am. Chem. Soc., 1975, v. 97, № 3, p.513-517.
77. Patent US 4113453. Apparat dlya nizkotemperaturnogo pryamogo ftorirovaniya. / Lagov R.J, Adkok I.L., Marashin N.I. –Publ. 12.09.78, RZh Khimiya, 1979, 19I130.
78. Patent US 4281119. Apparat dlya pryamogo ftorirovaniya s kontroliruemym ohlazhdeniem po zonam. / Lagov R.J., Adkok I.L., Marashin N.I. –Publ. 28.07.81, RZh Khimiya, 1982, 9N225.
79. Rahimov A.I., Himiya i tekhnologiya ftororganicheskih soedinenij. –M.: Khimiya, 1986, p.9-10.
80. Schmeisser M., Ehlers K. P., Sartori P. Dichlorhexafluorpropan durch fluorierung von 1,2-dichlorpropan. –Angew. Chem., 1967, v.79, № 13, p.622.
81. Margrave W. K. R., Smith F. Organic Fluorides. PartІ. Fluorination of hydrocarbons. –J. Chem. Sоc., November, 1949, p.3021-3026.
82. Grosse A.V., Keidi J. H. Svojstva ftoruglerodov. V kn. «Himiya ftora»: № 1 –M.: IL, 1950, p.35-64.
83. Patent GB 1281822. Improved Fluorination Process. / Kingdom R. J., Bond G. D. –Publ. 19.07.72.
84. Hill M. Process and market development of fluorocarbon fluids. –Chem.аnd Ind., 1975, № 3, p.118-121.
85. Patent FR 2028457 (V). Sposob ftorirovaniya. / Imperial Smelting Korp. –Publ. 15.01.70, Off. Bull. Francii, Himiya i metallurgiya, 1970, № 45-48, v.І, p.44.
86. Patent US 4220606. Sposob polucheniya perftorproizvodnyh iz ciklichekih uglevodorodov. / Moore R.E. –Publ. 02.09.80, RZh Khimiya, 1981, 9N105.
87. Patent US 3480667. Method of producing fluorinated compounds. / Siegart W. R., Blackley W. D. –Publ. 25.11.69.
88. Patent US 4330475. Aehrozol'nyj sposob pryamogo ftorirovaniya i ustrojstvo dlya ehtoj celi. / Adkok D.L., Renk E.B. –Publ. 18.05.82, RZh Khimiya, 1983,ІІN76.
89. Ruff J. K. The catalytic fluorination of perfluorocarbon nitriles and imines. –J. Org. Chem., 1967, v.32, № 5, p.1675-1677.
90. Lusting M., Ruff J. K. Fluorination of some perfluoro alkyliminosulfurdifluorides. –Inorg. Chem., 1965, v.4, № 10, p.1444-1446.
91. Fokin A.V., Stolyarov V.P., Radchenko V.P. Sintezpoliftoramino-iα-ftornitrosoedinenij na osnove reakcij gazoobraznogo ftora. –Izv. AN SSSR, ser. khim., 1982, № 8, p.1853-1861.
92. Fokin A.V., Galahov V.S., Uzun A.T. i dr. Reakciya ftora s solyami shchelochnyh metallov dinitroacetonitrila v prisutstvii ftoridov kaliya ili kal'ciya. –Izv. AN SSSR, ser. khim., 1974, № 2, p.456-458.
93. Fokin A.V., Uzun A.T., Stolyarov V.P. Bis (diftoramino) ftoracetal'degid –novyj predstavitel'α,α-bis (diftoramino) al'degidov. –Izv. AN SSSR, Ser. khim., 1982, № 6, p.1438.
94. Eremenko L.T., Nacibullin F.YA., Borovinskaya I.P., Karpova N.D. Sintez perftornitroehtanov. –Izv.AN SSSR, Ser.khim., 1968, № 3,p.431-432.
95. Sekiya A., Des Martean D. D. Synthesis of 1,1-bis (fluoroxy) –perhaloalkanes by reaction of fluorinated carboxylic acids with fluorine in the presence of cesium fluoride. –Inorg. Chem., 1980, v. 19, № 5, p.1328-1330.
96. Lu S., Des Martean D. D. Direct synthesis of fluorinated peroxides. 7. Perfluoro-tret-butyl-fluoroformyl peroxide. –Inorg. Chem., 1978, v. 17, № 2, p.304-306.
97. Schack C. J. A new synthesis of difluoraminotrifluoromethane. –J. Fluor. Chem., 1981, v. 18, no 4, p.583-586.
98. Patent DE 2712732. Sposob polucheniya oktaftorpropana. /Halaz S.P. –Publ. 28.09.75, RZh Khimiya, 1979, 14N14.
99. Patent US 4158023. Sposob polucheniya oktaftorpropana. / Halaz S.P. –Opubl. 12.06.79. Izobreteniya v SSSR i za rubezhom, 1979, v. 55, № 24, p.121.
100. Patent GB 1568020.Sposob polucheniya oktaftorpropana. / Halaz S.P. –Publ. 21.05.80. Izobreteniya v SSSR i za rubezhom, 1981, v. 55, № 3, p.54.
101. Patent US 4377715. Poluchenie perftorpropana. /Nuchka H.R., Hino I.B., Abek R.E., Robinson N.A. –Publ. 22.03.83, Izobreteniya v SSSR i za rubezhom, 1983, v.57, № 23, p.88.
102. Patent EU 0031519. Sposob ftorirovaniya organicheskih soedinenij ehlementarnym ftorom. / Ellaid Chemical Co. –Publ. 08.07.81, Izobreteniya v SSSR i za rubezhom, 1983, v.57, № 1, p.20.
103. Patent EU 0032210. Sposob ftorirovaniya organicheskih soedinenij ftorom v trubchatom reaktore iz poristogo metalla v prisutstvii perftorirovannogo razbavitelya. / Ellaid Chemical Co. –Publ. 22.07.81, Izobreteniya v SSSR i za rubezhom, 1983, v.57, № 2, p.27.
104. Patent US 4513154. Sposob provedeniya posledovatel'no-konkuriruyushchih gazofaznyh reakcij. / Kurtz B.E. –Publ. 23.04.85, RZh Khimiya, 1986, 4N25.
105. Patent US 2831035. Proizvodstvo ftorirovannyh uglerodov. / Tuchkovskij E.A., Wolf C. –Publ. 15.04.58, RZh Khimiya, 1960, 85740.
106. Patent US 3709800. Poluchenie perftorirovannyh uglevodorodov./ Fox H.M. –Publ. 09.01.73, RZh Khimiya, 1973, 22N23.
107. Патент Японии 5608. Фторсодержащие галоидуглеводороды. / Осиба Такаси. –Опубл. 22.03.65, РЖ Химия, 1968, 5Н35.
108. Patent US 2681267. Process uluchsheniya kataliticheskih svojstv ftoristogo alyuminiya i produktov iz nego. / Kalfee J. D., Miller Ch.B. –Publ. 15.06.54, RZh Khimiya, 1956, № 14, 45553.
109. Hohorst F. A., Shreeve J. M. Bis-(fluoroxy)-difluoromethane,CF2(OF)2. –J. Am. Chem. Soc., 1967, v.89, № 8, p.1809-1819.
110. Walker N., Des Martean D. D. Direct Synthesis of fluorocarbon peroxides.ІІІ. The addition of chloroperoxytrifluoromethane to olefins. –J. Am. Chem.Soc., 1975, v.97, № 1, p.13-17.
111. Patent US 4499024. Nepreryvnyj sposob polucheniya bis (ftoroksi) diftormetana. / Filolt M.G. –Publ. 12.02.85, RZh Khimiya, 1985, 20N14.
112. Lusting M. A., Pitochelli A. R., Ruff J. K. The catalytic addition of fluorine to a carbonyl group. Preparation of fluoroxy compounds. –J. Am. Chem. Soc., 1967, v. 89, № 12, p.2841-2843.
113. Ruff J. K., Pitochelli A. R., Lusting M. A. A simple synthesis of fluoroxyperfluoroalkyl compounds. -J. Am. Chem. Soc., 1966, v.88,№ 19, p.4531-4532.
114. Kennedy R. C., Cady G. H. Reaction of carbonyl fluoride with fluorine in the presence of various fluorides as catalysts. –J. Fluor. Chem., 1973, v.3, № 1, p.141-154.
115. Muhametshin F. M. Uspekhi himii ftororganicheskih gipogalogenitov i rodstvennyh soedinenij. –Uspekhi khimii, 1980, t. 49, № 7, p.1260-1288.
116. Muhametshin F.M. Gipoftority i ih primenenie v organicheskom sinteze. –V kn.«Novye ftoriruyushchie reagenty v organicheskom sinteze». –Novosibirsk: Nauka, 1987,p.140-196.
117. Cady G. H. Proposed mechanisms of catalysis of fluorination of CF2O. –Anales. Asoc. Quim. Argentina, 1971, v.59, p.3-4, p.125-131.
118. Patent US 3230264. Reaction of carbonyl fluoride with fluorine. –Roger S. P., Cady G.H. –Publ. 18.01.66.
119. Wechsberg M., Cady G.H. Comparative studies of catalytic fluorination of carbon monoxide with elementary fluorine. –J. Am. Chem. Soc., 1969, v.91, p.4432-4435.
120. Kellog K. B., Cady G.H. Trifluoromethyl hypofluorite. –J. Am. Chem. Soc., 1948, v.70,№ 12, p.3986-3988.
121. Gervasi J.A., Brown M., Bigelow L. A. The action of elemental fluorine upon organic compounds. XX. The fluorination of mono-, di-and trimethylamine, ethylenediamine and ethyleneimine. –J. Am. Chem. Soc., 1956, v.78, № 8, p.1679-1682.
122. Avonda F. P., Gervasi J. A., Bigelow L.A. The action of elemental fluorine upon organic compounds. XXІ. The fluorination of malononitrile and dimethylformamide. –J. Am. Chem. Soc., 1956, v.78, № 12, p.2798-2800.
123. Toitelboim M.A., Shoihet A.A., Kaplunov M.G., Vedeneev V.I. Energeticheskie razvetvleniya cepej v reakciyah triftormetilgipoftorita s galoidmetanami. –Kinetika i kataliz, 1981, t. 22, v. 2, p. 298.
124. Toitelboim M.A., Shoihet A.A., Vedeneev V.I. Energeticheskie razvetvleniya cepej v reakciyah triftormetilgipoftorita s galoidmetanami. 2. Mekhanizm otricatel'nogo vzaimodejstviya cepej. –Kinetika i kataliz, 1981, t. 22, v. 3, p. 564-568.
125. Vedeneev V.I., Parijskaya A.V. Mekhanizm ftorirovaniya metana i ego ftorproizvodnyh. І. Sravnenie skorostej ftorirovaniya metana, ftormetana, diftormetana i triftormetana. –Kinetika i kataliz, 1971, t. 12, v. 1, p. 21-26.
126. Parijskaya A.V., Vedeneev V.I. Mekhanizm ftorirovaniya metana i ego proizvodnyh. 2. Diftormetan –Kinetika i kataliz, 1971, t.12, v.2, p.293-298.
127. Parijskaya A.V., Vedeneev V.I. Mekhanizm ftorirovaniya metana i ego ftorproizvodnyh. 3. Ftoristyj metil. –Kinetika i kataliz, 1971, t.12, v.3, p.543-548.
128. Parijskaya A.V., Vedeneev V.I. Mekhanizm ftorirovaniya metana i ego ftorproizvodnyh.4. Metan. –Kinetika i ataliz, 1971, t. 12, v.4,p. 839-842.
129. Nadtochenko V.A., Fedotov N.B., Vedeneev V.I., Sarkisov O.M. O reakcii kolebatel'no vozbuzhdennoj molekuly CH3F s ftorom. –Dokl. AN SSSR, 1978, t.238, № 6, p.1391-1394.
130. Parijskaya A.V., Vedeneev V.I. O prirode zaderzhek samovosplameneniya v sisteme CH3F + F2 + O2 + He. –Kinetika i kataliz, t.14, v.6, p.1365-1369.
131. Vedeneev V.I., Teitelboim M.A., Shoihet A.A. Fotohimicheskoe ftorirovanie ftoroforma v prisutstvii kisloroda. –Izv. AN SSSR, 1976, № 9, p.1968-1970.
132. Medvedev B.A., Teitelboim M.A., Shilov A.E. Himicheskaya aktivaciya molekuly CHFCl2 v reakcii CHCl2+ F2→ CHCl2F + F . –Kinetika i kataliz, t.12, v.2, p. 269-275.
133. Medvedev B.A., Teitelboim M.A., Shilov A.E. Mekhanizm gazofaznoj reakcii hlorftormetana s molekulyarnym ftorom. –Kinetika i kataliz, 1971, t.12, v.3, p. 49-751.
134. Vedeneev V.I., Medvedev B.A., Teitelboim M.A. Gazofaznoe ftorirovanie diftormetana pri povyshennyh davleniyah inertnogo gaza. –Kinetika i kataliz, t.13, v.1, p.50-53.
135. Obvivalneva A.A., Fedotov V.G. Vozbuzhdennye molekuly v reakcii ftora s acetonom (ftoracetony; sintez; mekhanizm). –Kinetika i kataliz, 1981, t.22, v.5, v.1095-1099.
136. Kapralova G.A., Margolina E.M., Rusin L.Y., Chajkin A.M., Shilov A.E. Rol' kolebatel'no-vozbuzhdennyh molekul v reakciyah ftorirovaniya molekulyarnym ftorom. –V sb. «5 Mezhdunar. simpozium po himii ftora», Tezisy dokl., M., : Nauka, 1969, p.102-104.
137. Knunyats I.L, Gambaryan N.P., Rohlin E.M. Karbeny. (Soedineniya dvuhvalentnogo ugleroda, promezhutochno obrazuyushchiesya v organicheskih reakciyah). –Uspekhi khimii, 1958, t.27, v.12, s.1361-1436.
138. Muhametshin F.M., Zhirnov O.M., Ainagos N.A., Petrov Y.I., Lyapunov M.I. O nekotoryh osobennostyah gipoftoritnogo metoda polucheniya perftormetilvinilovogo ehfira. Soobshch. 2. Sintez triftormetilgipoftorita. –Tr. GIPH, № 69, inv. № T-2769, L., 1980, p.73-78.
139. Muhametshin F.M., Povroznik S.V., Zhirnov O.M. Issledovanie kinetiki i mekhanizma kataliticheskoj reakcii obrazovaniya triftormetilgipoftorita. –Tr. GIPH, № 103, inv. № T-2981, L., 1983, p.75-80.
140. Patent SU 1141707. Sposob polucheniya 1,1-diftor-1-hlorehtana ili 1,1,2,2-tetraftordihlorehtana. /Zaharov V.Y., Goldinov A.L., Borovnev L. M., Golubev A.N., Kalashnikova N.A., Zaharova O.M., Lyubimova L.A.
141. Kapralova G.A., Buben S.N., Chaikin A.M. Kinetika i mekhanizm reakcii ftorirovaniya okisi ugleroda. –Kinetika i kataliz, 1975, t.16, v.3, p.591-595.
142. Povroznik S.V. Razrabotka sposoba i tekhnologii polucheniya ftoroksitriftormetana reakciyami ehlementarnogo ftora s karbonilsoderzhashchimi soedineniyami. Diss. kand. tekhn. nauk, 1987, PF NPO GIPH, 161p.
143. Krasnov K.S. Molekuly i himicheskaya svyaz'. –M.: Vyssh. shkola, 1977, p.117.
144. Bezmelicyn V.N., Legasov V.A., Chaivanov B.B. Sintez diftorida kriptona s primeneniem termicheskoi generacii potokov atomarnogo ftora. –Dokl. AN SSSR. Ser. khim., 1977, t.235, № 1, p.96-98.
145. Bezmelicyn V.N., Legasov V.A., Spirin S.N., Chajvanov B.B. Kinetika kataliticheskoi atomizacii ftora. –Dokl. AN SSSR. Ser.fiz.khimiya, 1982, t.262, № 5, p.1153-1157.
146. Palkina L.A., Spirin S.N., Tishchenko P.P. processy s uchastiem atomarnogo ftora. –Dokl. AN SSSR. Ser.fiz.khimiya, 1982, t.262, p.1428-1433.
147. Bezmelicyn V.N., Vasilev A.A., Sinyanskij V.F., Chajvanov B.B. Kinetika termokataliticheskoj dissociacii ftora na poverhnosti nikelya. –Tezisy dokladov na 8 Vsesoyuzn. simp. po himii neorg. ftoridov, 1987, M.: Nauka, p.59.
148. Nikonorov YU.I. Ftoridy nikelya. Ih kataliticheskaya i okislitel'naya aktivnost'. –Tezisy dokladov na 5 Vsesoyuzn. simp. po himii neorg. ftoridov, 1978, M.: Nauka, p.205.
149. Ryss I.G. Khimiya ftora i ego neorganicheskih soedinenij. –M.: Goskhimizdat, 1956, p.576-579.
150. Redwood M.E., Willis C. J. Fully fluorinated alkoxides. PartІ. Trifluoromethoxides of alkali metals. –Canad. J. Chem., 1965, v.43, p.1893-1898.
151. Gubanov V.A., Veretennikov N.V., Troichanskaya P.E., Dolgopol'skij I.M. Sintez perftoralkoksidov metallovІ gruppy i termograficheskoe izuchenie ih svojstv. –Zh. Org. khim., 1975, t.ІІ, № 2, p.322-325.
152. Levy J. B., Kennedy R. C. Bis (trifluoromethyl) peroxide.І. Thermodynamics of the equilibrium with carbonyl fluoride and trifluoromethyl hypofluorite. –J. Am. Chem. Soc., 1972, v.94, no 10, p.3302-3305.
153. Patent JP 35888. Sposob polucheniya triftormetilgipoftorita. / Nagase Syundzi, Abe Takasi, Baba Hadzime, Ohira Kadzuo. –Publ. 09.09.72, RZh Khimiya, 1973, 14N120.
154. Patent US 3687825. Sposob polucheniya triftormetilgipoftorita. Nagase Syundzi, Abe Takasi, Baba Nadzime. –Publ. 29.08.72, RZh Khimiya, 1973, 21N26.
155. Patent US 2983764. Sposob polucheniya ftoruglerodnyh soedinenij./ Knak D.F. –Publ. 9.05.61, RZh Khimiya, 1962, 14L53.
156. Patent JP 56-16429. Oligomerizaciya geksaftorpropilena v gazovoj faze. / Osaka Yonosuke, Higasidzuka Takehsi. –Publ. 17.02.81, RZh Khimiya, 1982, 3N10.
157. Patent JP 50-45818. Poluchenie oligomerov geksaftorpropilena. Kadzava Masahiro, Komatsu Tadaaki, Macuoka Kimiaki. –Publ. 08.06.81, RZh Khimiya, 1982, 9NІІ.
158. Patent US 4296265. Sposob polucheniya oligomerov geksaftorpropilena. / Osaka Yonosuke, Tozuka Takasi. –Publ. 20.10.81, RZh Khimiya, 1982, І3NІІ.
159. Patent DE 3027229. Sposob polucheniya oligomerov geksaftorpropilena. / Osaka Y., Tozuka T. –Publ. 07.02.81, Izobreteniya v SSSR i za rubezhom, 1981, V.55, № 12, p.30.
160. Patent BE 2055824A. Sposob polucheniya oligomerov geksaftorpropilena. /Osaka Y., Tozuka T. –Publ. 18.07.80, Sbornik referatov NIOKR, obzorov, perevodov i dep. rukopisej. DSP. Khimiya i khimicheskaya tekhnologiya, M., 1986, № 5, p.14.
161. Patent DE 2616733. Sposob polucheniya di- i trimerov geksaftorpropilena. / Okava M., Komatsu T., Matsuoka K. –Publ. 20.03.80, Izobreteniya v SSSR i za rubezhom, 1980, № 15, p.13.
162. Patent JP 57-2697. Sposob polucheniya oligomerov geksaftorpropena. / Neosu K.K. –Publ. 18.01.82, Izobreteniya v SSSR i za rubezhom, 1982, v.57, № 14, p.113.
163. Scherer K. V., Ono T., Jamanouchi K., Fernandez R., Henderson P.B. F-2,4-Dimethyl-3-ethyl-3-pentyl and F-2,4-dimethyl-3-isopropyl-3pentyl: stable tert-perfluoroalkyl radicals prepared by addition of fluorine or trifluoromethyl to a perfluoroalkene. –J. Am. Chem. Soc., 1985, v.107, № 3, p.718-719.
164. Scherer K. V., Fernandez R., Henderson P.B. Long lived perfluoroalkyl radicals: preparation by direct fluorination and alternate routes, properties and rates andmechanisms of disappearance. –Int. Symp. “Centenary of the discovery of fluorine”, Abstr., Paris, 1986, p.1.
165. Patent DE 2332097. Sposob polucheniya perftornonanov. /Halaz S.P. –Publ. 16.01. 75, RZh Khimiya, 1975, 18N25.
166. Patent DE 2332088. Sposob polucheniya perftor-2-metilpentana./ Halaz S.P. –Publ. 16.07.81, Izobreteniya v SSSR i zarubezhom, 1981, v.55, № 23, p.21.
167. Allayarov S.R., Barkalov I.M., Gol'danskij V.I., Kiryuhin D.P. Obrazovanie stabil'nyh radikalov iz ftororganicheskih soedinenij. –Izv. AN SSSR. Ser. him., 1983, № 6, p.1225-1228.
168. Allayarov S.R., Barkalov I.M., Gol'danskij V.I., Demidov S.V., Kiryuhin D.P., Mihajlov A.I. Stabil'nye radikaly –rastvorennye perftoralkily. –Izv. AN SSSR. Ser. him., 1983, № 6, p.1448-1449.
169. Allayarov S.R., Mihailov A.I., Barkalov I.M. Novyj stabil'nyj perftoralkil'nyj radikal. –Izv. AN SSSR. Ser. him., 1985, № 7, p.1667-1669.
170. Allayarov S.R., Barkalov I.M., Lebedov M.YU., Loginova N.N., Mihailov A.I. Obrazovanie stabil'nyh radikalov pri radiolize geksaftorpropilena. –Izv.AN SSSR. Ser. him., 1986, № 2,p.462-463.
171. Allayarov S.R., Barkalov I.M., Gol'danskij V.I. Obrazovanie dolgozhivushchih radikalov pri ftorirovanii zhidkih uglevodorodov. – Izv.AN SSSR. Ser. him., 1986, № 10, p.394.
172. Allayarov S.R., Sumina I.V., Barkalov I.M., Mihailov A.I. Obrazovanie stabil'nyh radikalov pri fotolize perftor-2,4-dimetil-3-ehtilpentena-2. –Izv. AN SSSR, Ser. him., 1986, № 10, p.2359-2361.
173. Krusic P. J., Scherer K. V. An electron spin resonance study of long lived fluoroalkyl radicals generated by photolysis of hindered perfluoroalkenes. Int. Symp. “Centenary of the discovery of fluorine”. Abstr., Paris, 1986, p.37.
174. Levy J.B., Kennedy R. C. Homolytic displacement reactions of carbon.І. The fluorine-perfluorocyclobutane reactions. –J.Am.Chem.Soc., 1974, v.96, № 15, p.4791-9795.
175. Gol'dinov A.L., Zaharov V.Y., Baibakov P.Y., ZHukova V.A., Novikova M.D. Novye aspekty pryamogo ftorirovaniya. Kataliticheskoe gazofaznoe ftorirovanie ftorolefinov; poluchenie perftoruglerodov. –Otchet predpriyatiya p/ya A-1619, 1987, inv. № 1288, 87p.
176. Matveev S.A., Prokudin L.P., Timofeev N.V. Ispol'zovanie triftorida kobal'ta dlya ftorirovaniya organicheskih soedinenij po dvojnym svyazyam. –Tr. GIPH, 1975, № 31, inv. № T-1728, p.119-127.
177. Asovich V.S., Prokudin I.P. Ftorirovanie acetilena ftoridami kobal'ta i ceriya. Tr. GIPH, № 55, inv. № T-2552, L., 1978, p.21-24.
178. Asovich V.S., Prokudin I.P., Maksimov B.N., Stepanov V.P. Izuchenie kineticheskih zakonomernostej ftorirovaniya acetilena trekhftoristym kobal'tom. –Tr. GIPH, № 65, inv. № T-2723, L., 1979, p.42-47.
179. Kotikov S.V., Prokudin I.P., Asovich V.S. Izuchenie kineticheskih zakonomernostej ftorirovaniya geksaftorpropilena trekhftoristym kobal'tom. –Tr. GIPH, № 101, inv. № T-2986, L., GIPH, 1985, s.24-27.
180. Burdon J., Knights J.R., Parsons J. W., Tatlow J. C. The fluorination of ethane and ethene over potassium tetrafluorocobaltate (ІІІ) and cobalt trifluoride. –Tetrahedron, 1976, v.32, p.1041-1043.
181. Gol'dinov A.L., Borovnev L.M., SHirokova N.S., Kovbasyuk O.G., Tishina V.V. Vliyanie tekhnologicheskih parametrov sinteza i chistoty rastvoritelya na svojstva ftoroplasta-50. –Otchet predpriyatiya p/ya A-1619, 1987, inv. № 1231.
182. Patent DE 2451493. Sposob polucheniya perftorirovannyh ehfirov./ Halaz S.P., Klag F. –Publ. 06.05.76, Izobreteniya v SSSR i za rubezhom,1982, v.57, № 22, p.5.
183. Patent US 3665041. Perfluorinated polyethers and process for their preparation. / Sianesi D., Fontanelli R., -Publ. 23.05.72.
184. Ryabinin N.A., Dolubenov A.E., Shmatov L.E. Ftorpoliehfiry kak antiadgezivy pri pressovanii perhlorata nitroniya. –Tr. GIPH, № 101, inv. № T-2986, L., 1985, p.83-87.
185. Patent SU 1332758. Sposob ochistki geksaftorpropilena, tetraftorehtilena i ih semej ot oktaftorizobutilena. /Fokin A.V., Studnev Y.N., Rapkin A.I. i dr.
186. Gol'dinov A.L., Borovnev L.M., Golubev A.N., Zaharov V.Yu. i dr. Razrabotka tekhnologii polucheniya 2-perftormetil-4-oksaperftornonana na osnove perftorizobutilena i 1,1,5-trigidroperftor pentanola. –Otchet predpriyatiya p/ya A-1619, 1987, inv. № 1229, 39p.
187. Patent SU 1148285. 3-Gidropropilftorsul'fat v kachestve promezhutochnogo produkta dlya sinteza 2,2,3,3-tetraftorpropionovoj kisloty i sposob ee polucheniya. /Fokin A.V., Studnev Y.N., Rapkin A.I. i dr.
188. Patent SU 3966753. Sposob polucheniya 2-gidrotetraftorehtilftorsul'fata. /Fokin A.V., Rapkin A.I., Tatarinov A.S. i dr.
189. Patent SU 1233595. Sposob polucheniya ω-gidroperftorkarbonovyh kislot (ego varianty). / Fokin A.V., Studnev Y.N., Rapkin A.I. i dr.
190. Tekhnicheskie trebovaniya k PFDMCG dlya ispol'zovaniya v radioehlektronike. M.: INEOS AN SSSR. Reg. № 12111-10 DSP ot 18.01.85.
191. Iskhodnye dannye dlya razrabotki zhidkosti-diehlektrika. M.: INEOS AN SSSR. Pril. k vh.№ 155 ot 18.02.85.
192. Gol'dinov A.L., Zaharov V.YU., Bajbakov P.YA. i dr. Razrabotka tekhnologii polucheniya perftordimetilciklogeksana povyshennoj chistoty. –Otchet predpriyatiya p/ya A-1619, 1986, inv. № 1218, 24p.
193. Gol'dinov A.L., Maslyakov A.I., Zaharov V.Y. i dr. O vozmozhnosti rezkogo uvelicheniya vypuska deficitnyh ftoruglerodnyh zhidkostej i smazok. –Otchet predpriyatiya p/ya A-1619, 1986, № 1219, 13p.
194. Vremennyj tekhnologicheskij reglament proizvodstva perftordekalina.1981, inv. № 1115.
195. Furin G.G. Synthetic aspects of the fluorination of organic compounds. (Ed.) M.E. Vol’pin./Harwood Academic
Publishers GmbH: United Kingdom, Sov. Sci. Rev., B. Chem., 1991, V.16, Part 1,р.140.
196. Rozen S., Kol M. Olefin epoxidation using elemental fluorine. / J.Org.Chem., 1990. V.55,р.5155-5159.
197. Patent JP 02-131438, (1990). CO719/08. Preparation of hexafluoroethane. /Shimuzu M.C.A. 1990, V.I 13,97031.
198. Patent JP 62-193946, (1989). CO719/08, CO17/04. Sposob polucheniya ftorsoderzhashchih ehtanov. /Faruka Y., Kono H. RZh Kh, 1990, 2N 131.
199. Lagow R.J. Important new developments in direct fluorination technology. /Memorial symposium for Prof. Nobuo Ishikawa, Dec. 9-10, 1991, Nippon Kaiun Club, Japan.
200. Lin W.H., Lagow R.J. Synthesis of perfluorodicyclohexano-crown-6 ether. /J.Chem.Soc., Chem.Commun., 1991,р.13-14.
201. Clark W.D., Lin T.Y., Maleknia S.D., Lagow R.J. Synthesis of perfluorotetralkyl ortocarbonates using elemental fluorine. / J.Org.Chem., 1989. V.54.р.1990-1992.
202. Sievert A.C., Tong W.R., Nappa M.J. Preparation D’analogues fluores de la pheromone d’anthanomus grandis. / J.Fluorine Chem., 1991. V.51.р.397-405.
203. Tonelli C., Tortelli V. Photoinduced fluorination of hexafluoropropene trimers: synthesis of branched perfluoroalkanes. /J.Chem.Soc., Perkin Trans I, 1990, № 1,р.23-26.
204. Patent Application EP 344935, (1989). CO8G65/00. Preparation of prefluoroethers by photo-assisted fluorination. / Nappa M.J., Sievert A. C.A. 1990, V. 112, 216229.
205. Patent US 4960951, (1990). CO743/12. Novel perfluoroalkyl ethers by two-step fluorination of polyols./ Nappa M.J., Sievert A.C. Tong W.R. C.A. 1991, V. 111, 216229.
206. Sievert A.C., Tong W.R., Nappa M.J. Synthesis of perfluorinated ethers by an improved solution phase direct fluorination process. / J.Fluorine Chem. 1991. V. 53,р. 397-417.
207. Mizin G.G., Simakov B.V., Shultezkaya L.V., Moldavsky D.D. Issledovanie sposoba polucheniya perftorehtilciklogeksena. / ZHPH. 1991. t.64 ,v.6. p.293-1296.
208. Moldavsky D.D., Mizin G.G., Shultezkaya L.V., Simakov B.V., Furin G.G. Synthesis of perfluoroethylcyclohexene, its alkoxy and diakylamino derivates and their electrochemical fluorination. / Abstracts of 10-European Symposium on Fluorine Chemistry, Sept. 20-25, 1992, Padua, Italy. Abstracts. B7; J. Fluorine Chem., 1992. v.58.р.185.
209. Moldavsky D. D. Perftororganicheskie soedineniya: Poluchenie pryamym ftorirovaniemuglevodorodov. Diss. dokt. him. nauk, 2002, RNC Prikladnaya himiya, 268p.
210. Suvorov B.V., Bukeihanov N.R. Okislitel'nye reakcii v organicheskom sinteze. 1978g. M., Khimiya, 197p.
211. Semenova G.A., Leites I.L., Aksel'rod Y.V., Markina M.I., Sergeev S.P.,Har'kovskaya E.N. Kataliticheskie i adsorbcionnye metody ochistki gazov ot sernistyh soedinenij. –Ochistka tekhnologicheskih gazov.1977g, M.,Khimiya, 1977,p. 35-37.
212. Panov G.I. Zakonomernosti geterogenno-kataliticheskoj aktivacii dvuhatomnyh molekul (H2, O2, N2). Novye kataliticheskie sistemy aktivacii molekulyarnogo azota. Diss. dokt. him. nauk, 1986, Novosibirsk, 374p.
213. Patent SU 220740. Sposob polucheniya tetrahlortetraftorpropana. –Palleta O., P. Svoboda, L. Stefan, Y. Kvisala. –Publ. 15.12.85. RZhKhimiya, 1986, ІІN24.
214. Patent JP 60-116637. Poluchenie ftormetana. –Takayama S., Meiraku F., Takoiti A., Kovasaki H. –Publ. 24.06.85. RZh Khimiya, 1986, 9N16.
215. Margolis L.Y. Okislenie uglevodorodov na geterogennyh katalizatorah. 1977g. M., Khimiya, p. 296.
216. Kavaev M.M., Zaporin A.P., Kleshchev N.F. Kataliticheskoe okislenie ammiaka. 1983g. M., Khimiya, p. 31.
217. Tekhnologiya katalizatorov. 1974g. L., Himiya, p. 138-140.
218. Patent US 2760997. Process for the production of chlorotrifluoroethylene by passing a mixture of trichlorotrifluoroethane and hydrogen through an unobstructed iron tube. / Rucker J. T., Stormon D. B. –Publ. 28.08.56.
219. Patent US 2615925. Preparation of olefinic compounds. / Bordner C.A. –Publ. 28.10.52.
220. Patent US 2704777. Preparation of halogenated olefines / Clark J.W. –Publ. 22.03.55.
221. Patent GB 698386. Improvement in preparation of halogenated olefines / Clark J. W. –Publ. 14.10.53.
222. Patent DE 1044800. Verfahren zur Herstellung von Chlortrifluorathylen / Clark J. W. –Publ. 27.11.58.
223. Patent US 2685606. Preparation of halogenated olefines / Clark J. W. –Publ. 03.08.54 C.A. 48, 127881 (1954).
224. Patent US 2697124. Dehalogenation of fluorohalocarbons / Russel M.M. –Publ. 14.12.54 C. A. 50, 2650 (1956).
225. Patent US 2864873. Production of 1,1,2-trifluoro-2-chloroethylene / Miller C. B., Smith L. B. –Publ. 16.12.58.
226. Patent US 3043889. Poluchenie ftoristogo ehtilena. / Smith L.B., Wolf C. –Publ. 10.07.62. RZh Khimiya, 1964, 3N24.
227. Patent DE 1186847. Poluchenie 1-chlor-1,2,2-triftorehtilena. / Smit L.B., Wolf C. –Publ. 4.10.65, RZh Khimiya, 1966, 13N26.
228. Patent US 3333011. Poluchenie hlortriftorehtilena./Anello L.G., Wolf C. –Publ. 25.07.67 RZh Khimiya, 1969, 14N30.
229. Patent JP 60-185734. Poluchenie triftorhlorehtilena. / Morimoto Takesi, Morikava Sanesuke, Funayama Keidi. –Publ. 21.09.85, 19N37.
230. Patent EU 0053657. Sposob polucheniya hlortriftorehtilena i triftorehtilena. / Kaningham U.D., Plekors R.E., Smith E.M. –Publ. 16.06.82. Izobr. v SSSR i z/r., vyp. 57, № 15, p. 83.
231. Patent JP 43-8454. Sposob polucheniya triftorehtilena. / Sakata Hirosukeh. –Publ. 02.04.68. RZh Khimiya, 1969, 3N36.
232. Patent US 2913400. Katalizator iz okisi molibdena dlya konversii uglevodorodov. / Burton U.P., Lefrenkois F.A., Riblet E.U. –Publ. 17.11.59, RZh Khimiya, 1961, 9K156.
233. Patent US 3354233. Proizvodstvo tetraftorehtilena. / Anello L.G., Wolf C. –Publ. 21.11.67, RZh Khimiya, 1969, 9N32.
234. Patent DE 1270547. Verfahren zur Herstellung von Tetrafluorathylen/ Anello Z. G., Wolf C. –Publ. 30.01.69.
235. Patent US 3505417. Degaloidirovanie ftorgalogenuglerodov. / Gardner L.E. –Publ. 07.04.70, RZh Khimiya, 1971, 11133.
236. Patent US 3636173. Sposob kataliticheskogo degaloidirovaniya./ Gardner L.E. –Publ. 18.01.72, RZh Khimiya, 1972, 21N21.
237. Patent US 3789016. Katalizator gidrodegaloidirovaniya. / Gardner L.E. –Publ. 29.01.74. RZh Khimiya, 1975, 2N217.
238. Patent US 3636172. Degaloidirovanie ftorgaloiduglevodorodov. / Gardner L.E. –Publ. 18.01.72, RZh Khimiya, 1972, 21N20.
239. Patent US 2900423. Proizvodstvo perftorpropilena. / Smith L.B. –Publ. 18.08.59. RZh Khimiya, 1961, 7L44.
240. Patent US 2734090. Poluchenie ftoristogo vinilidena. / Kafi D.D., Miller CH.B. –Publ. 07.02.56, RZh Khimiya, 1957, 12, 42313.
241. Patent US 3404180. Poluchenie carbonilftorida. / K.K. Lester. –Publ. 1.10.68. –RZh Khimiya, 1969, 23N92.
242. Patent FR 1460246. Sposob polucheniya okisi tetraftorehtilena. / Edison. –Publ. 17.10.66. –RZh Khimiya, 1968ІІNІ24.
243. Sokolov L.F. Issledovanie i razrabotka tekhnologii processov okisleniya ftorolefinov. –Diss. dokt. khim. nauk. –Leningrad, VNIISK, 1979.
244. Patent SU 190555. Sposob polucheniya ftoristogo vodoroda. / Gol'dinov A.L., Romanov E.I., Zaharov V.Y. i dr.
245. Gol'dinov A.L., Borovnev L.M., Zaharov V.Y. i dr. Termicheskoe obezvrezhivanie ftororganicheskih othodov v vodorodo-vozdushnom plameni na opytno-promyshlennoj ustanovke. –Otchet predpriyatiya p/ya A-1619, 1982, inv. № 887, 18p.
246. Borovnev L.M., Golubev A.N., Zaharov V.Y. i dr. Kataliz i iniciirovanie destruktivnogo okisleniya, tetraftorehtilena molekulyarnym kislorodom. Poluchenie karbonilftorida. –Otchet predpriyatiya p/ya A-1619, 1983, inv. № 1110, 21p.
247. Patent DE 213342. Sposob polucheniya ftorsoderzhashchih galoiduglevodorodov so srednej stepen'yu ftorirovaniya. / L. Ingehburg, K. Rehjnfred, M. Diter. –Publ. 24.12.85. –RZh Khimiya, 1986, 21N17.
248. Patent DE 203314. Katalizator polucheniya hlorftormetana v gazovoj faze. / I. Lelid, R. Kaden, D. Mross. –Publ. 19.10.83. –RZh Khimiya, 1984, 17N181.
249. Patent US 4504686. Sposob polucheniya 1,1,2-triftor-2-hlorehtildiftormetilovogo ehfira. / S. Takami. –Opubl. 12.03.85. –RZH Himiya, 1986, 8N9.
250. Patent JP 55-85564. Poluchenie β-triftormetilhlorpiridinov./ R.Nishima, K. Rudzikama, I.Jokomiti, Y. Cudzii, S. Nisimura. –Publ. 27.06.80. –RZh Khimiya, 1981, 19N177.
251. Patent US 4139568. Sposob polucheniya metilftorida. / V. Daniehl, M. Libbs. –Publ. 13.02.79. –RZh Khimiya, 1979, 18N18.
252. Patent US 4145368. Poluchenie 1,1,1-triftor-2,2-dihlorehtana. R. Sweney, S. Bernard. –Publ. 20.03.79. –RZh Khimiya, 1979, 20N20.
253. Patent FR 1453510. Sposob ochistki dihlortetraftorehtana. / Corp. Elektrohimiya. –Publ. 16.08.66. –RZh Khimiya, 1968, 11N39.
254. Vilenchik Y.M., Yakurnova G.I., Ostapenko E.A. Sintez triftoracetilftorida na osnove okisi tetraftorehtilena. –Tr. GIPH, 1985, № 101, p. 147-149.
255. Patent JP 58-35978. Sposob polucheniya pentaftorpropionilftorida. / A. Jonotasi, A. Josio. –Publ. 08.05.83.-Izobreteniya v SSSR i za rubezhom, 1984, v. 57, № 8, part 2, p. 130.
256. Patent JP 58-38231. Poluchenie pentaftorpropionilftorida./ I. Jonosuke, A. Josio, S. Hiroyuki. –Publ. 05.03.83.-RZh Khimiya, 1984, 5N46.
257. Patent US 3250808. Ftorsoderzhashchie prostye ehfiry. / E.F. Mur, A. Mil'yan, G. Eleuterio. –Publ. 10.05.66. –RZh Himiya, 1967, 17N82.
258. Patent US 3250807. Ftorsoderzhashchie karbonovye kisloty s ehfirnymi gruppirovkami. / CH.G. Fritz, E.F. Moore. –Publ. 10.05.66. –RZh Khimiya, 1967, 17N84.
259. Patent US 3242218. Sposob polucheniya ftoruglerodnyh poliehfirov. / V.Miller. –Publ. 22.03.66. –RZh Khimiya, 1967, 12S232.
260. Patent DE 78376. Sposob polimerizacii i sopolimerizacii okisi geksaftorpropilena v prisutstvii geksaftorpropilena. / G. Lothar. –Publ. 12.12.70. –RZh Khimiya, 1971, 21S361.
261. Patent US 3125599. Polimery okisej ftoruglerodnyh soedinenij. / D. Vornell. –Publ. 17.03.64. –RZh Khimiya, 1965, 15S277.
262. Patent US 3291843. Fluorinated vinilethers. / Fritz C.G., Selman S., -Publ. 13.12.66.C.A. 1967, 66, 37427 h.
263. Patent US 4377717. Sposob polucheniya perftor-2-metilpentena-2. / L.Anello, R. Sweeny. –Publ. 22.03.83. –RZh Khimiya, 1983, 24N1311.
264. Knunyats I.L., Fokin A.V., Cheburkov Y.A. Chetvertyi mezhdunarodnyj simpozium po himii ftora v Istes-Park (Kolorado, USA) –ZHVHO im. D.I.Mendeleeva, t. 13, № 3, p. 311-321.
265. Patent US 3449389. Pervichnye ftorzameshchennye alkogolyaty. / D. Vornell. –Publ. 10.06.69. –RZh Khimiya, 1970, 18N39.
266. Patent US 3322826. Polimerizaciya geksaftorpropilenehpoksida./ E.F.Moore –Publ. 0.06.67. –RZh Khimiya, 1968, 18S 296.
267. Patent JP 57-45132. Poluchenie perftor-2-metil-3-oksageksanoilftorida. / Y. Masaki, M. Sehjdzi. –Publ. 13.03.82. –RZh Khimiya, 1983, 5N70.
268. Patent US 3274293. Ftorsoderzhashchie prostye ehfiry. / S.Stanley. –Publ. 20.09.66. –RZh Khimiya, 1968, 5N161.
269. Patent DE 2756919. Sposob demerizacii okisi geksaftorpropilena. / G. Kuhne, F. Geller. –Publ. 05.07.79. –RZh Khimiya, 1980, 14N22.
270. Patent DE 2924385. Sposob dimerizacii okisi geksaftorpropilena. / G. Kuhne. –Publ. 07.08.80. –RZh Khimiya, 1981, 23N36.
271. Kinle H., Bader E. Aktivnye ugli i ih promyshlennoe primenenie. –L., Khimiya, 1984, p. 185-192.
272. Patent US 3321515. Sposob polucheniya ftorirovannyh karbonil'nyh soedinenij. / F.Moore, S.Milyan. –Publ. 23.05.67.-RZh Khimiya, 1968, 15N48.
273. Bekker R.A., Asratyan G.V., Dyatkin B.L. O roli prostranstvennyh faktorov v reakciyah poliftorirovannyhα-okisej s aminami. –ZhOrKh, 1973, t. 9, v. 8, p. 1644-1648.
274. Knunyants I.L., Shokina V.V., Tuleneva, V.V., Ceburkov Y.A., Aronov Y.E. Proizvodnye perftorizomaslyanoj kisloty. –Izv. AN SSSR, ser. khim., 1966, № 10, p. 1831-1833.
275. Sianesi D., Passety A., Tarli F., The Chemistry of hexafluoropropene epoxide. –J. Org. Chem., 1966,v. 31, № 7,p. 2312-2316.
276. Borovnev L.M., Golubev A.N., Zaharov V.Y. i dr. Iniciirovanie processa sopolimerizacii tetraftorehtilena i geksaftorpropilena perftorirovannymi diacil'nymi perekisyami, sintezirovannymi na osnove okisi geksaftorpropilena. –Otchet predpriyatiya p/ya A-1619, 1985, inv. № 1154, 28p.
277. Ashmor. Kataliz i iniciirovanie himicheskih reakcij. M.: Mir, 1966, p. 62.
278. Patent US 3213134. Deciklizaciya ftorirovannyh ciklicheskih efirov. / D. Morin. –Publ. 19.10.65. –RZh Khimiya, 1967, 9N60.
279. Patent US 4302608. Sposob izomerizacii okisi geksaftorpropilena do geksaftoracetona. / E.Skuri, D. Milz. –Publ. 24.11.81. –RZh Khimiya, 1982, 20N56.
280. Patent JP 58-62131. Poluchenie geksaftoracetona. / O. Josio, A. Josimasa, M. Makisuke. –Publ. 13.04.83. –RZh Khimiya, 1984, 12N30.
281. Patent JP 58-62130. Poluchenie geksaftoracetona. / A. Josimasa, M. Makisuke, S. Yukio. –Publ. 13.04.83. –RZh Khimiya, 1984, 12N29.
282. Patent SU 740741. Sposob polucheniya geksaftoracetona. / Y.M. Vilenchik, G.I. Yakurnova, G.I. Lekomceva, L.P. Zayakina, A.P. Harchenko. –Publ. 15.06.80. –RZh Khimiya, 1980, 24N67.
283. Patent US 859348. Sposob polucheniya geksaftoracetona. / Y.M. Vilenchik, G.I. Yakurnova. –Publ. v B.I., 1981, № 32, RZh Khimiya, 1982, 21N12.
284. Patent US 4238416. Sposob izomerizacii ftorirovannyh soedinenij. / D. Koduo. –Publ. 09.12.80. –RZh Khimiya, 1981, 17N65.
285. Kiperman S.L. Vvedenie v kinetiku geterogennyh kataliticheskih reakcij. M.: Nauka, 1964, p. 401.
286. Tyul'ga G.M., Gubanov V.A., Popova I.S., Petruhno L.A., Bolhovec B.M., Troichanskaya P.E., Dolgopol'skij I.M. Izuchenie reakcii ftorangidridov perftorkarbonovyh kislot s ftoridami shchelochnyh metallov. –ZhOrKh, 1973, t. 14, v. 11, p. 2339-2346.
287. Konshin A.I., Gubanov V.A. Ob ocenke ehffektivnosti sposoba ochistki hladonovyh rastvorov perftorpolioksametilenacetilftoridov. –Otchet predpriyatiya p/ya V-8415.
288. Patent SU 188404. Ftorsopolimer vinilidenftorida s 3,6,8,10,12-pentaoksaperftortridecenom dlya termomorozoagressivostojkih izdelij. / Grejs A.M., Ershov A.E., Zaharov V.Y. i dr.
289. Karcov S.V., Valov P.I., Sokolov L.F., Sokolov S.V. Rol' poverhnosti reakcionnogo sosuda v processe zhidkofaznogo okisleniya geksaftorpropilena. –Izv. AN SSSR, ser. khim., 1978, № 10. p. 2268-2272.
290. Patent SU 229185. Sposob polucheniya perftorpolioksametilenacetilftoridov. / Zaharov V.Y., Gol'dinov A.L., Borovnev L.M. i dr.
291. Borovnev L.M., Golubev A.N., Zaharov V.Y. i dr. Dinamika izmeneniya sostava gazovoj i zhidkoj faz v promyshlennom reaktore sinteza okisi geksaftorpropilena v hode operacii okisleniya. –Otchet predpriyatiya p/ya A-1619, 1983, inv. № 1089, 10p.
292. Borovnev L.M., Golubev A.N., Zaharov V.Y. i dr. Dinamika obrazovaniya perftorpolioksametilen acetilftoridov v processe zhidkofaznogo okisleniya geksaftorpropilena kislorodom na promyshlennoj ustanovke. –Otchet predpriyatiya p/ya A-1619, 1985, inv. № 1165, 22p.
293. Gol'dinov A.L., Borovnev L.M., Zaharov V.Y. i dr. Okislenie geksaftorpropilena molekulyarnym kislorodom v prisutstvii 1,2-dibromtetraftorehtana (hladona-114V2). Poluchenie okisi geksaftorpropilena. –Otchet predpriyatiya p/ya A-1619, 1982, inv. № 1072, 57p.
294. Patent SU 1128436. Plamyagasyashchaya smes'. / Borovnev L.M., Gusenkov M.V., Zhukova V.A. i dr.
295. Kravchenko N.N., Popov V.A., Ershov Y.A., Pomeranceva E.G. Nekotorye osobennosti fotoliza perftorirovannyh poliehfirov. –VMS, t. 23, № 3, 1981, p. 180-183.
296. Patent SU 3011822. Sposob regeneracii hladona-113. / Panitkova E.S., Berenblit V.V., Senyushov A.N. i dr.
297. Zejfman Y.V., Ter-Gabrielyan E.G., Ganbaryan N.P., Knunyantc I.L. Himiya perftorizobutilena. –Uspekhi khimii, 1984, t. 53, v. 3, p. 431-461.
298. Knunyantc I.L., Shokina V.V., Kuleshova A.D. Prisoedinenie galoidvodorodov k ftorolefinam. –Izv. AN SSSR, ser. khim., 1960, № 9, p.1693-1695.
299. Postovoi S.A., Mysov E.I., Zeifman Y.V., Knunyantc I.L. Nukleofil'naya kondensaciya oktaftorizobutilena s tetraftorehtilenom. –Izv. AN SSSR, ser. khim., 1982, № 7, p. 1586-1590.
300. Patent US 3389187. Dimer perftorizobutilena. / D.L. Miller. –Publ. 18.06.68. –RZh Khimiya, 1969, 17N3211.
301. Knunyantc I.L., German L.S., Dyatkin B.P. Reakcii ftorolefinov. SoobshchenieІІ. Vzaimodejstvie soedinenij ryada perftorizobutilena s aminami i ammiakom. –Izv. AN SSSR, otd.him.n., 1960, № 2, p. 221-230.
302. Patent JP 53-73504. Udalenie oktaftorizobutilena. / Kuroda Takehsi, Furukava Josiki, Macuoha Nu. –Publ. 30.06.78. RZh Khimiya, 1979, 10N2011.
303. Dorogimskij V.A., Kolomiec A.F., Sokol'skij G.A. O reakcii oktaftorizobutilena s ehtilenglikolem i glicerinom. –ZhOrKh, 1983, t.19, v. 8, p.1762-1763.
304. Patent US 129653. Sposob polucheniya geksaftorizomaslyanoj kisloty. / Knunyantc I.L., Cheburkov Y.A., Krasuskaya M.P. –RZh Khimiya, 1961, 9L75.
305. Borovnev L.M., Golubev A.N., Zaharov V.Y. i dr. Promyshlennye ispytaniya i vnedrenie sposoba ochistki gazov piroliza tetraftorehtilena ot perftorizobutilena. –Otchet predpriyatiya p/ya A-1619, 1981, inv. № 1048, 34p.
306. Gol'dinov A.L., Borovnev L.M., Zaharov V.Y. i dr. Uvelichenie stepeni ispol'zovaniya syr'ya v proizvodstve geksaftorpropilena putem bolee polnogo izvlecheniya oktaftorciklobutana iz kubovoj frakcii. –Otchet predpriyatiya p/ya A-1619, 1982, inv. № 1063, 16p.
307. Patent SU 180854. Sposob polucheniya geksaftorpropilena. / Borovnev L.M., Vinogradova A.P., Golubev A.N. i dr.
308. Borovnev L.M., Golubev A.N., Zaharov V.Y. i dr. Kompleksnaya pererabotka kubovoj frakcii proizvodstva tetraftorehtilena. –Otchet predpriyatiya p/ya A-1619, 1985, inv. № 15/107 DSP, 17p.
309. Patent SU 1336492. Sposob polucheniya 2-hlortetraftorehtilftorsul'fata. / Fokin A.V., Rapkin A.I., Tatarinov A.S. i dr.
310. Patent SU 1184808. Sposob polucheniya monoftorsul'fata broma. / Fokin A.V., Studnev Y.N., Rapkin A.I. i dr.
311. Patent SU 1239092. Sposob polucheniya tris(ftorsul'fata) broma. / Fokin A.V., Studnev Y.N., Rapkin A.I. i dr.
312. Patent SU 3982041. 3-(Poliftorpropionamido) propil-2-oksiehtildimetilammonij hloridy, obladayushchie gipotenzivnym dejstviem. / Fokin A.V., Kovalev G.V., Mazanov L.S. i dr.
313. Patent SU 3982040. N-Poliftoracil'nye proizvodnyeγ-amnomaslyanoj kisloty, obladayushchie gipertenzivnym dejstviem. / Fokin A.V., Kovalev G.V., Muhina N.V. i dr. Pol. resh. ot 26.09.86.
314. Patent SU 1334655. 3-(ω-Hlorperftoralkanoilamido)propil-dimetil-2-oksiehtilammmonij hloridy v kachestve poverhnostno-aktivnyh veshchestv. /Fokin A.V., Rapkin A.I., Studnev Y.N. i dr.
315. Patent SU 196359. Sposob polucheniyaω-hlorperftorkarbonovyh kislot i ehmul'gator. / Fokin A.V., Studnev Y.N., Rapkin A.I. i dr.
316. Patent DE 3034549. Sposob polucheniya perftorirovannyh ftorangidridov karbonovyh kislot. / Verner S. –Publ. 29.04.82, –RZh Khimiya, 1983, 17N5711.
317. Promyshlennye katalizatory i nositeli. Spravochnik. –Novosibirsk, 1972, p. 246.
318. T. Van der Plas. Tekstura i himiya poverhnosti uglerodnyh tel. V kn.: Stroenie i svojstva adsorbentov i katalizatorov. –M.: Mir, 1973, p. 436-441.
319. Patrik S. Uspekhi khimii ftora. –M., L., Khimiya, 1964, t.1, № 2, p. 336-379
Recommended for publication by prof. S. M. Igoumnov
Fluorine Notes, 2014, 97, 1-2
