Received: November, 2014
Fluorine Notes, 2014, 97, 7-8
Synthesis of Perhalogenated tert-Butyl-α-fluoroacrylates and their Optic Characteristic Data
A.A. Tyutyunovabc, V.E. Boykoabc, A.V. Sin’koabc, S.M. Igumnovabc, S.I. Molchanovac, E.V. Khaidukovc, V.I. Sokolovc
a A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences,
Vavilov St. 28, GSP-1, Moscow, Russian Federation
e-mail: tuytuynov@rambler.ru
a JS SIA "P&M-Invest", Leninsky prospekt, 47, 119334 Moscow, Russia
c The Institute on Laser and Information Technologies of the Russian Academy of Sciences (ILIT RAS), 1 Svyatoozerskaya St. 140700 Shatura, Moscow Region, RUSSIA
Abstract: New perhalogenated tert-butyl-α-fluoroacrylates like CH2=CFCO2R, where R = (CF3)3C, CF2Cl(CF3)2C, CF3(CF2Cl)2C are synthesized. Their absorption and refractive spectra in telecommunication fields of wave lengths are around 0.85, 1.3 and 1.55 μm. It is stated, that monomers’ coefficient of absorption at 1.55 μm doesn’t exceed 0.1 dB/cm, refractive index is within the range of 1.2990–1.3586.
Keywords: α-fluoroacrylates, coefficient of absorption, refractive index.
Optical devices based on polymer materials are of significant interest for creating of high-speed data communication/transmission lines [1-2]. Polyacrylates are of most specific attention among the whole variety of polymers. It is due to both the simplicity of synthesis and modification of acrylic monomers and their ability to polymerize under the influence of UV radiation, what makes their use possible to create integrated optical devices using methods of photolithography and direct laser drawing [3-5].
Polyacrylates used for production of optical cores and optical wave guides shells must be of high glass transition temperature (Ts > 110°C), thermal stability (Td > 200°C), optical transmittancy/transparency (< 0.07 dB/cm around 1.55 μm), the fluctuations of refractive index with temperature increase of these polyacrylates must be insignificant (dn/dT < 5.10-5), these polyacrylates must be mechanically durable and flexible at the same time, resistant to environmental impact [6-9]. Different methacrylates or fluoroacrylates containing poly-halogenated alkyl and/or aryl groups in the ether part of the molecule are used to obtain polymers which possess such properties [10-22].
It is known, that polymers of the least C-H bond concentration are of highest optical transmittancy in telecommunication fields of wave lengths [4-5, 8-9, 11]. It is obvious, that among acrylic monomers the ethers of α-fluoroacrylic acids obtained on the basis of perhalogenated tertiary alcohols are of particular interest for obtaining polyacrylates of maximum high optical transmittancy and maximum high photopolymerizing activity among polymers of the present type. The development of synthesis methods of perhalogentaed tert-butyl-α-fluoroacrylates which had not been described earlier and the determination of their optical characteristics are the task of the present article.
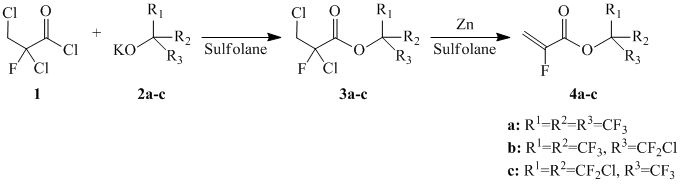
The sytnthesis of α-floroacrylates 4a-c has been executed according method [7] using the reaction of chloroanhydride (1) with alcoholytes of tertiary alcohols 2a-c with further dechlorination of forming esters 3a-c (Refer to Scheme 1):
Scheme 1
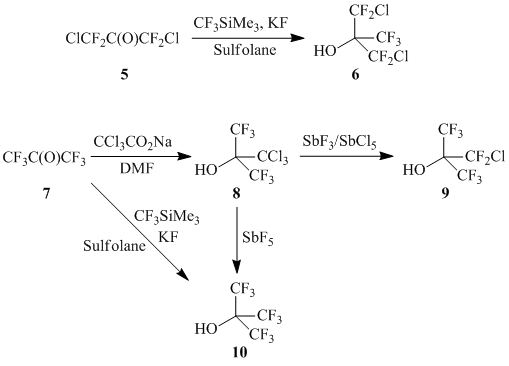
Alcohols 6, 9, 10 have been described earlier [23-24]; their obtaining scheme is listed below (Refer to Scheme 2):
Scheme 2
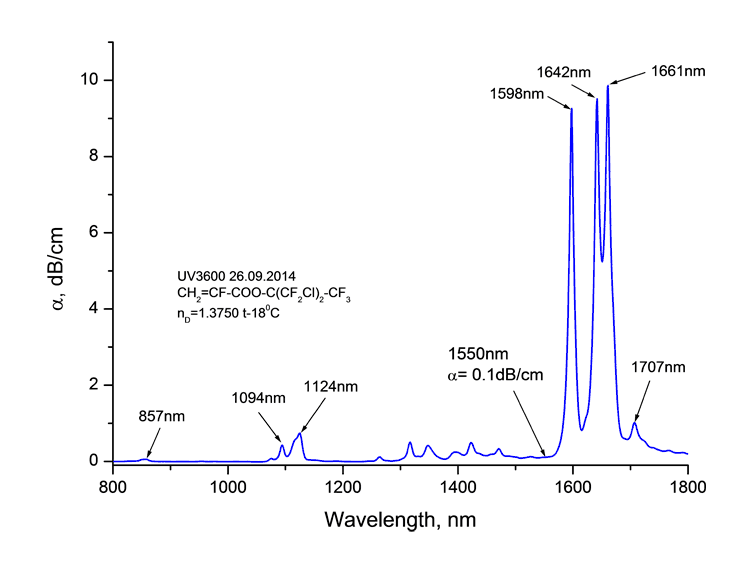
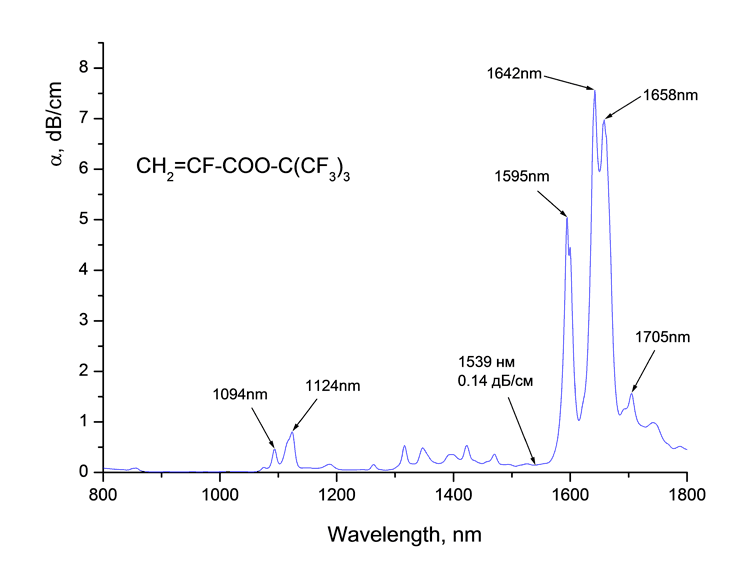
Absorption spectra of synthesized monomers in telecommunication ranges of wave length around 0.85, 1.3 and 1.55 μm measured at spectrophotomer Shimadzu UV3600 are listed at Picture 1. Absorption bands are observed in spectra as well as the windows of transmittancy. The coefficient of absorption of monomers around 1.55 μm is 0.1 dB/cm.
|
|
 b) b) |
|
|
Pic.
1. Monomers absorption spectra CH2=CF-CO2C(CF3)3 (a), CH2=CF-CO2C(CF2Cl)(CF3)2 (b)
and CH2=CF-CO2C(CF2Cl)2(CF3) (в) around
1.55 μm.
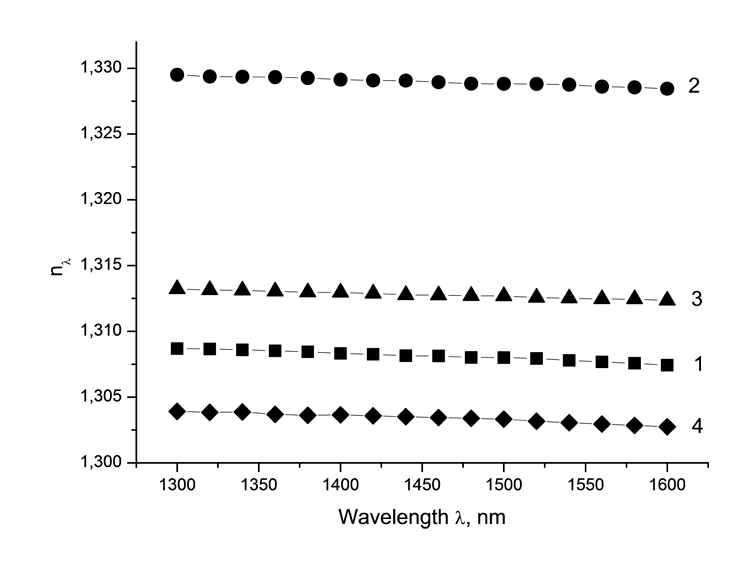
Dispersion dependencies of monomers’ refractive index are measured at spectroscopic refractomer [25] created ILIT RAS (Refer to Pic 2). Dispersion of monomers are usual, their refractive index at wave length λ=1.55 μm is 1.2990 (4a), 1.3288 (4b) up to 1.3586 (4c), and is increasing as number of chlorine atoms goes up.
|
|
Pic. 2. Refractive indices nλ of halogenated acrylic monomers CH2=CF-CO2C(CF3)3 (1), CH2=CF-CO2C(CF2Cl)(CF3)2 (2), CH2=CF-CO2C(CF2Cl)2CF3 (3) in a neighboring IR field of spectrum measured at 20°C.
Synthesized monomers are capable of radical photopolymerization and film forming. They can be used to form polymer wave guides and other elements of integrated optical devices using methods of contact UV photolithography and direct laser drawing.
Experimental Part
NMR 1H, 19F spectra are registered at spectrometer “Bruker AVANCE-300” at 300 and 282 MHz, respectively, exterior standard CDCl3. Chemical shifts for 1H spectra are listed relative to residual signal of solvent (δ 7.26) and are given in ppm relative to TMS. Chemical shifts of spectra 19F are listed in ppm relatively to CFCl3. Downfield shift are positive.
Synthesis of compounds 1, 6, 9, 10 has been described earlier [7, 23-24]. Alcoholytes of tertial alcohols 2a-c are obtained according to known method [26].
Esters 3a-c are obtained by interaction of chloroanhydride 1 and alcoholytes 2a-c in sulfpholane at 20-50°C, with further distillation of reaction’s product from the reaction mixture in vacuum 10-15 Torr and additional purification 3a-c by distillation.
1,1,1,3,3,3-Hexafluorine-2-(trifluoromethyl)propan-2-yl 2,3-dichlorine-2-fluoropropanoate (3a). Boiling point. 50-52°C(15 Torr). NMR 1H δ: 4.0÷4.2 (m, CH2Cl); NMR 19F δ: -125.4 (m, 1F, CFCl), -71.7 (m, 9F, CF3).
1-Chloro-1,1,3,3,3-pentafluoro-2-(trifluoromethyl)propan-2-yl 2,3-dichlorine-2-fluoropropanoate (3b). Boiling point. 67-68°C(10 Torr). NMR 1H δ: 3.8÷4.1 (m, CH2Cl); NMR 19F δ: -125.4 (m, 1F, CFCl), -70.0 (s, 6F, CF3), -59.5 (s, 2F, CF2Cl).
1,3-Dichlorine-1,1,3,3-tetrafluoro-2-(trifluoromethyl)propan-2-yl 2,3-dichlorine-2-fluoropropanoate (3c). Boiling point 200-205°C. NMR 1H δ: 3.8÷4.1 (m, CH2Cl); NMR 19F δ: -124.5 (m, 1F, CFCl), -67.2 (m, 3F, CF3), -56.8 (m, 4F, CF2Cl).
Perhalogenated Tert-Butyl-α-Fluoroacrylates 4a-c Common Synthesis Method.
The esters 3a-c (0.1 mole) is added drop by drop while stirring to the suspension of zinc dust (9,81g, 0.15 g-atom) activated by adding of trimethylchlorosilane (1 ml) in sulpholane (50 ml) in vacuum 10-15 topp at 30-40°C, the reaction product is distilled into container cooled to 0°C. The obtained product is additionally rectified in vacuum 10-15 Torr. The yield of purified product is 70-75%. The purity of obtained monomers according to GLC is 98-99%.
1,1,1,3,3,3-Hexafluorine-2-(trifluoromethyl0propan-2-yl 2-fluoroacrylate (4a). CH2=CFCO2C(CF3)3. Boiling point 19-20°C(15 Torr). NMR 1H δ: 5.7÷6.2 (m, CH2=); NMR 19F δ: -119.4 (m, 1F, CFCl), -71.7 (m, 9F, CF3).
1-Chloro-1,1,3,3,3-pentafluoro-2-(trifluoromethyl)propan-2-yl 2-fluoroacrylate (4b). CH2=CFCO2C(CF3)2CF2Cl. Boiling point 33.5-34°C(10 Torr). NMR 1H δ: 5.5÷6.0 (m, CH2=); NMR 19F δ: -118.7 (m, 1F, CF), -69.6 (m, 6F, CF3), -59.1 (m, 2F, CF2Cl).
1,3-Dichlorine-1,1,3,3-terafluoro-2-(trifluoromethyl)propan-2-yl 2-fluoroacrylate (4c). CH2=CFCO2C(CF2Cl)2CF3. Boiling Point 62.5-63°C(10 Torr). NMR 1H δ: 5.5÷6.0 (m, CH2=); NMR 19F δ: -118.2 (m, 1F, CF), -67.1 (m, 3F, CF3), -56.7 (m, 4F, CF2Cl).
Acknowledgments.
Work has been executed due to the financial support of RSCF (Russian Scientific Foundation) grant 14-19-01659
References
- W. Daum, J. Krauser, P.E. Zamzow, O. Ziemann. POF – polymer optical fibers for data communication. ISBN 3-540-42009-6, Springer-Verlag Berlin Heidelberg New York, 2002.
- O. Ziemann, J. Krauser, P.E. Zamzow, W. Daum. POF Handbook – Optical Short Range Transmission Systems. 2nd Edition. ISBN 978-3-540-76628-5, Springer-Verlag Berlin Heidelberg, 2008.
- H. Ma, A.K.-Y. Jen, L.R. Dalton Adv. Mater., 2002, 14, 1339-1365.
- M. Zhou Optical Engineering, 2002, 41, 1631-1643.
- Y. Koike, K. Koike J.Pol.Sci: Part B: Pol.Phys., 2011, 49, 2-17.
- T. Kaino, Y. Katayama Pol.Engin. and Sci., Mid-Sept., 1989, 29, 1209-1214.
- L.S. Boguslavskaya, I.Yu. Panteleeva, T.V. Morozova, A.V. Kartashov, N.N. Chuvatkin Russ.Chem.Rev., 1990, 59, 906-917.
- B. Boutevin, A. Rousseau, D. Bosc J.Pol.Sci: PartA: Pol.Chem., 1992, 30, 1279-1286.
- A. Rousseau, B. Boutevin AIP Conf.Proc., 2004, 709, 214-232.
- Japan Patent 1974, №49-129545.
- T. Kaino J.Pol.Sci.: Part A: Pol.Chem., 1987, 25, 37-46.
- T. Sakagami, N. Murayama US Patent 1988, №4,775,590.
- H. Teng, L. Yang, F. Mikes, Y. Koike, Y. Okamoto Polym.Adv.Technol., 2007, 18, 453-457.
- A. Tagaya, T. Harada, K. Koike, Y. Koike, Y. Okamoto, H. Teng, L. Yang J.Appl.Pol.Sci., 2007, 106, 4219-4224.
- D. Zhou, H. Teng, K. Koike, Y. Koike, Y. Okamoto J.Pol.Sci.: Part A: Pol.Chem., 2008, 46, 4748-4755.
- K. Koike, F. Mikes, Y. Koike, Y. Okamoto Polym.Adv.Technol., 2008, 19, 516-520.
- K. Koike, F. Mikes, Y. Okamoto, Y. Koike J.Pol.Sci.: Part A: Pol.Chem., 2009, 47, 3352-3361.
- K. Koike, T. Kado, Z. Satoh, Y. Okamoto, Y. Koike Polymer, 2010, 51, 1377-1385.
- J.-M. Cracowski These Docteur, Universite du Maine, U.F.R. Sciences et Techniques, 2008.
- J.-M. Cracowski, V. Montembault, I. Hardy, D. Bosc, B. Ameduri, L. Fontaine J.Pol.Sci: Part A: Pol.Chem., 2008, 46, 4383-4391.
- J.-M. Cracowski, V. Montembault, D. Bosc, B. Ameduri, F. Odobel, L. Fontaine J.Pol.Sci:PartA:Pol.Chem., 2009, 47, 1403-1411.
- W. Yao, Y. Li, X. Huang Polymer, 2014, http://dx.doi.org/10.1016/j.polymer.2014.09.036.
- R.E.A. Dear Synthesis, 1970, 361-362.
- Yu.V. Zeifman Bull.Russ.Acad. of Sci., Div. of chem. sci., 1992, 41, 370-373.
- V.I. Sokolov, M.S. Kitai, G.V. Mishakov, S.I. Molchanova, V.Ya. Panchenko, I.V. Sokolova PTE, 2011, 157-158.
- F.J. Pavlik US Patent 1972, #3668233A.
Recommended for publication by Prof. S. R. Sterlin
Fluorine Notes, 2014, 97, 7-8
 a)
a)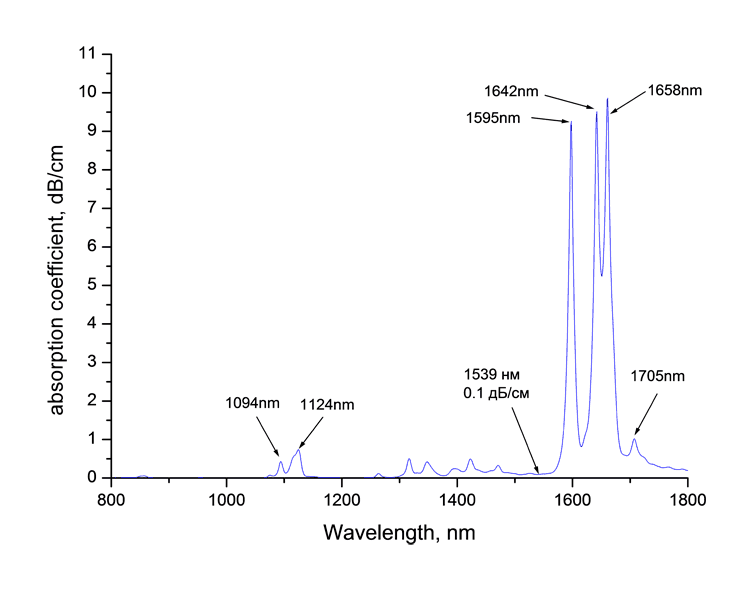 c)
c)