Received: November, 2014
Fluorine Notes, 2014, 97, 5-6
Optical Properties of Cyanoperfluoro– and Cyanochloroperfluoroalkylacrylates in “Data Communication” Field of Wave Length Around 0.85 μm
V.E. Boyko1, S.I. Molchanova2, A.V. Sin'ko1, V.I. Sokolov2, A.A. Tyutyunov1, E.V. Khaidukov2, S. M. Igoumnov1
1 A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Vavilov St. 28, GSP-1, Moscow, Russian Federation
2 The Institute on Laser and Information Technologies of the Russian Academy of Sciences (ILIT RAS), 1 Svyatoozerskaya St. 140700 Shatura, Moscow Region, RUSSIA
Abstract: A number of cyanoperfluoro- and cyanochlorineperfluoro-alkylacrylates with halogenation grade equal to 66.6% has been synthesized out of ketones. Monomers are transparent colorless liquids of low viscosity able to conduct radical photopolymerization. Monomers’ absorption and refraction spectra have been measured in “data communication” field of wave length around 0.85 μm. It has been proved, that monomers’ coefficient of absorption ranges from 0.04 to 0.09 dB/cm, refractive index lies within the range of 1.336 - 1.457. The synthesized monomers can be used to form polymer waveguides and other elements integrally – the optical devices using UV photolithography and direct laser drawing.
Keywords: Cyanoperfluoroalkylacrylates, cyanochlorinefluoroalkylacrylates, coefficient of absorption, refractive index, integrated optics.
Introduction
The synthesis of halogenated (fluorine and chlorine containing) monomers is one of the most intensive studied fields of organic and macromolecular chemistry. Among such monomers most attention is paid to the chemistry of fluorinated acrylates. Initially they were used as oleophobic and hydrophobic coatings in textile and leather industries. Further studies of fluoroacrylates based on primary alcohols like CH2=CH-COO-CH2-Rf, where Rf – perfluorinated radical have revealed another promising field of application – as transparent optical materials for priority direction of modern connection and information transferring technics - fibre and intergrated optics [1 - 11]. Good optic properties of these monomers and corresponding polymers in combination with high heat and moisture resistance, mechanical durability and at the same time flexibility cause their significant advantage compare to methacryltes (for example, poly(methyl methacrylate), PMMA). Nowadays many fluoroalkyl polymers of wide spectrum of refractive index have been synthesized and patented [2 - 11], that allows their using in creation of optical fibers and waveguides [12 - 16]. In terms of all said above it is advisable to synthesize and study acrylates containing tertiary alcoholic groups, and groups with one or few fluorines substituted for chlorine or cyano-groups.
Synthesis of Cyanoperfluoro- and Cyanochlorineperfluoroalkylacrylates
We have developed synthesis methods of halogenated (fluorine- and chlorine containing) monomers, we have produced cyanoperfluoro- and cyanochlorineperfluoroalkylacrylates, refer to Pic. 1. Monomers have been obtained by corresponding acetons reacting with sodium cyanide with further interaction with acroleilchloride, which led to formation of acrylates in demand. The present reaction is a single-stage process with two steps conducted in acetonitrile 0°C.
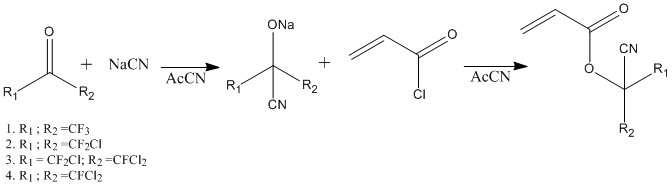
Pic. 1. Scheme of Cyanoperfluoro- And Cyanochlorineperfluoroalkylacrylates Synthesis.
Refractive indices nD and boiling points showings have been determined for all obtained monomers, refer to Table 1.
Table 1. Synthesized Cyanoperfluoro- And Cyanochlorineperfluoroalkylacrylates And Their Main Characteristics.
|
Monomer |
Halogenation Grade |
nD |
Boiling Point |
|
CH2=CH-COO-C(CF3)2-CN |
66.6% |
1.3417 |
127°C |
|
CH2=CH-COO-C(CF2Cl)2-CN |
66.6% |
1.4051 |
68-70°C at 20 mm Hg |
|
CH2=CH-COO-C(CF2Cl)(CFCl2)-CN |
66.6% |
1.4364 |
90-94°C at 20 mm Hg |
|
CH2=CH-COO-C(CFCl2)2-CN |
66.6% |
1.4685 |
116-120°C at 20 mm Hg |
Studying Optical Properties of Monomers Around to 0.85 μm
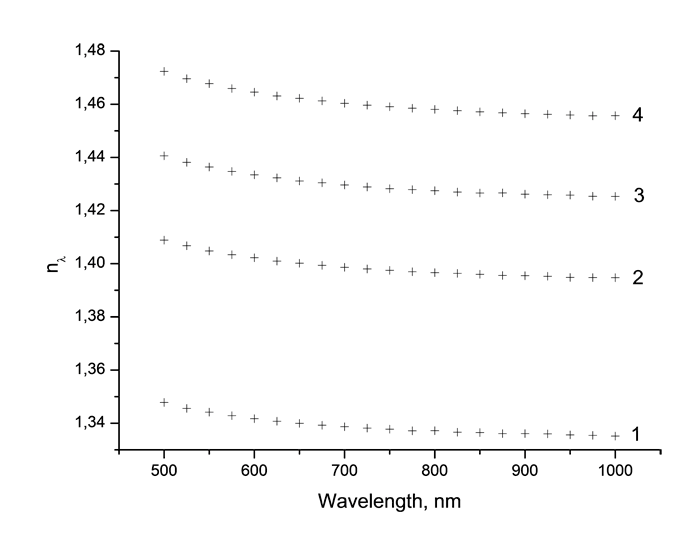
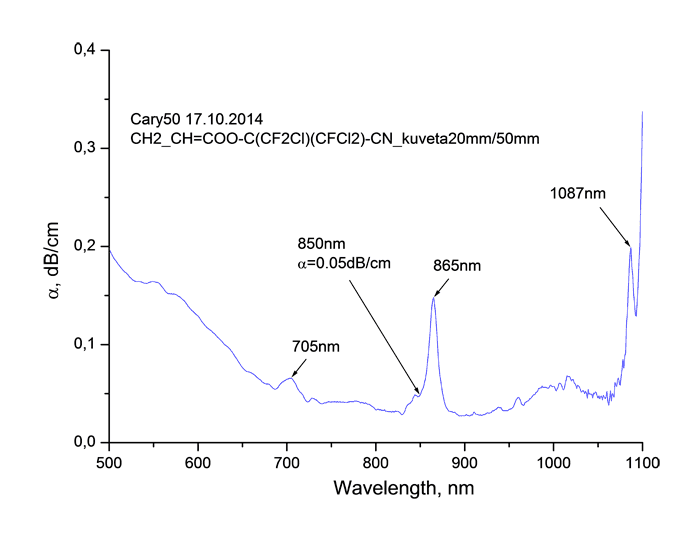
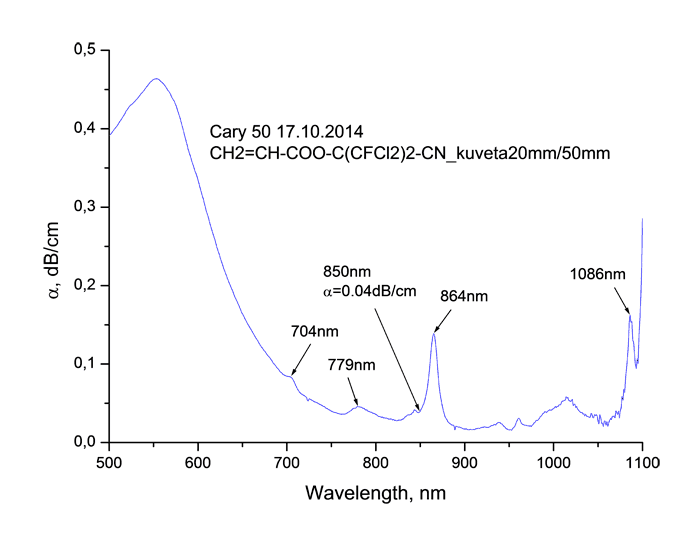
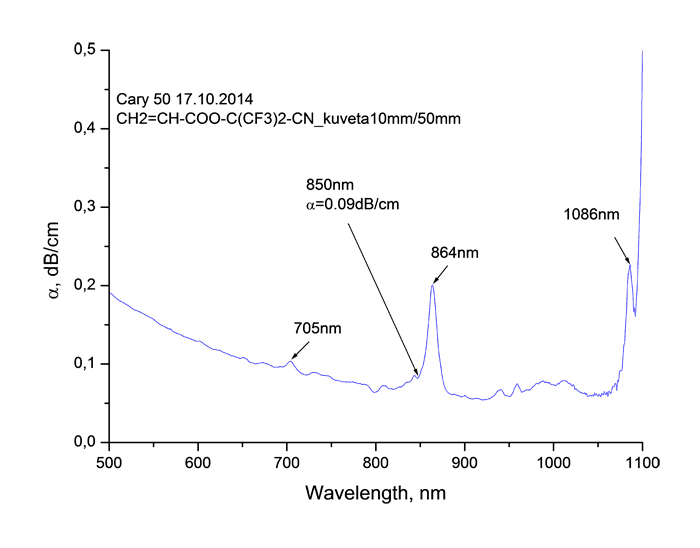
Absorption spectra of synthesized monomers in “data communication” field of wave length around 0.85 μm measured using spectrophotometer Cary-50 are listed at Pic. 2. It is obvious, that in spectra we observe the fringes of absorption caused by stretching vibrations of atoms consisting in molecule, as well as windows of transparency. Absorption lines with centres around 865 and 705 μm are caused by combination tones connected with double C=C bonds [17].
|
|
|
|
 c) c) |
 d) d) |
Pic. 2. Absorption spectrum of monomers CH2=CH-COO-C(CF3)2-CN (s), CH2=CH-COO-C(CF2Cl)2-CN (b), CH2=CH-COO-C(CF2Cl)(CFCl2)-CN (c), CH2=CH-COO-C(CFCl2)2-CN (d) in “data communication” field of wave length around 0.85 μm.
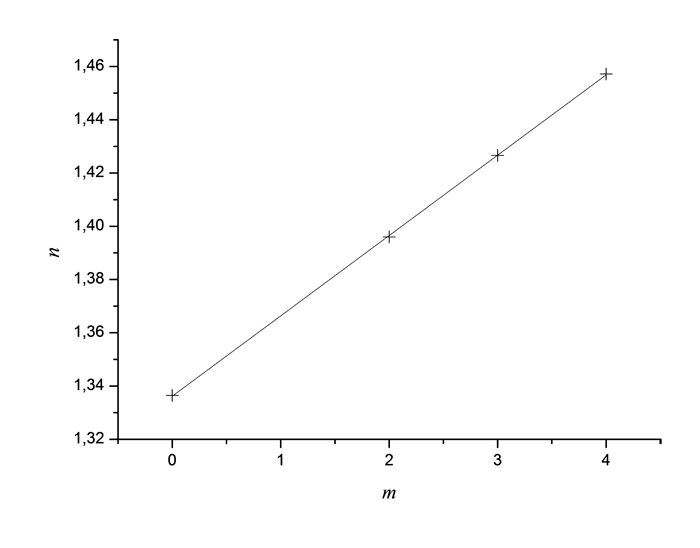
At Pic. 3 you will find dispersion dependencies of refractive index of these monomers measured using spectroscopic refractometer created in ILIT RAS [18]. It is obvious, that dispersion of monomers is normal (around 0.85 μm). Optical properties of synthesized monomers are summed in Table 2.
|
|
Pic. 3. Dispersion Dependencies of Monomers’ Refractive Index CH2=CH-COO-C(CF3)2-CN
(1), CH2=CH-COO-C(CF2Cl)2-CN (2), CH2=CH-COO-C(CF2Cl)(CFCl2)-CN
(3), CH2=CH-COO-C(CFCl2)2-CN (4) around 0.85 μm, measured
at spectroscopic refractometer at 20 °C.
Table 2. Optical Properties of Cyanoperfluoro- And Cyanochlorineperfluoroalkylacrylates At “Data Communication” Wave Length of λ = 0.85 μm.
|
Monomers |
Coefficient of Absorption, dB/cm |
Refractive Index |
Dispersion |
|
CH2=CH-COO-C(CF3)2-CN |
0.09 |
1.3365 |
-1.0•10-5/nm |
|
CH2=CH-COO-C(CF2Cl)2-CN |
0.05 |
1.3960 |
-1.3•10-5/nm |
|
CH2=CH-COO-C(CF2Cl)(CFCl2)-CN |
0.05 |
1.4266 |
-1.3•10-5/nm |
|
CH2=CH-COO-C(CFCl2)2-CN |
0.04 |
1.4572 |
-1.5•10-5/nm |
As you can see from Table 2 the coefficient of absorption of monomers at wave length of λ = 0.85
μm lies within the range of 0.04 - 0.09 dB/cm, the refractive index goes up from 1.336 to
1.457. The increasing of chlorination grade results in increasing of monomers’ refraction, refer
to Pic. 4.
|
|
Pic.4. The Dependency of Refractive Index n(λ = 0.85 μm) of Halogenated
Acrylates Listed in Table 4 on the amount of chlorine atoms m вin the monomer molecule (crosses).
Firm line – linear approximation by the method of the smallest squares.
Film Forming Properties of Monomers
The synthesized monomers are capable of radical photopolymerization and film forming. That is why they can be used to form polymeric wave guides and other elements of integrated optical devices using methods of UV photolithography and direct laser drawing [19].
Experimental Part
2-Cyano-1,1,1,3,3,3-hexafluoropropan-2-yl acrylate CH2=CH-COO-C(CF3)2-CN. To a three-neck flask of 500 ml volume 16g of sodium cyanide are added to 200 ml of dry acetonitrile while stirring, then the reaction mixture is cooled to 0°C and 50g of 1,1,1,3,3,3-hexafluoropropan-2-one are bubbled. The mixture is stirred 30 minutes more at 0°C and for 1,5 h at 20°C. Then the reaction mixture is cooled to 0°C and then 32,5g of propenoyl chloride are added drop by drop. All is mixed for 30 minutes more at 0°C and for 1,5h at 20°C. Then the reaction mixture is poured into water. The lower layer is separated, dried with magnesium sulphate, filtered and distilled in vacuum. 43g of 2-cyano-1,1,1,3,3,3-hexafluoropropan-2-yl acrylate are obtained in the form of colorless liquid. The yield is 58%. The purity is 98%. Boiling point = 127°C. Elemental analysis (calculated, %): C, 34.03; H, 1.22; F, 46.13; N, 5.67; elemental analysis (found, %): C, 34.0; H, 1.32; F, 46.06; N, 5.67.
2-Cyano-1,3-dichloro-1,1,3,3,-tetrafluoropropan-2-yl acrylate CH2=CH-COO-C(CF2Cl)2-CN. To a 500ml volume three-neck flask 19g of sodium cyanide are added to 200ml of dry acetonitrile while stirring, then the reaction mixture is cooled 0°C and 70g of 1,3-dichloro-1,1,3,3,-tetrapropan-2-one are added drop by drop. It is being mixed for 30 minutes more at 0°C and for 1,5 h at 20°C. Afterwards the reaction mixture is cooled down 0°C and 42g of propenoyl chloride are added drop by drop. It is being mixed for 30 minutes more at 0°C and for 1.5h at 20°C. Then the reaction mixture is poured into water. The lower layer is separated, dried with magnesium sulphate, filtered and distilled in vacuum. 85g of 1,3-dichloro-2-cyano-1,1,3,3-tetrafluoropropan-2-yl acrylate are obtained in the form of colorless liquid. The yield is 86%. The purity is 99.3%. Boiling point = 68-70°C / 20 mm Hg. Elemental analysis (calculated %): C, 30.03; H, 1.08; F, 27.14; N, 5.00; elemental analysis (found, %): C, 30.0; H, 1.1; F, 27.1; N, 5.06.
1,1,3-trichloro-2-cyano-1,3,3,-trifluoropropan-2-yl acrylate CH2=CH-COO-C(CF2Cl)(CFCl2)-CN. To a 500ml three-neck flask 24g of sodium cyanide are added to 250ml of dry acetonitrile while stirring, then the reaction mixture is cooled to 0°C and 98g of 1,1,3-trichloro-1,3,3,-trifluoropropan-2-one are added drop by drop. It is being mixed for 30 minutes more at 0°C and for 1,5h at 20°C. Afterwards the reaction mixture is cooled to 0°C and 55g of propenoyl chloride are added drop by drop. It is being mixed for 30 minutes more at 0°C and for 1,5h at 20°C. Afterwards the reaction mixture is poured into water. The lower layer is separated, dried with magnesium sulphate, filtered and distilled in vacuum. 99g of 1,1,3-trichloro-2-cyano-1,3,3-trifluoropropan-2-yl acrylate are obtained in the form of colorless liquid. The yield is 73%. The purity is 98.6%. Boiling point = 90-94°C / 20 mm Hg. Elemental analysis (calculated %): C, 28.36; H, 1.02; F, 19.23; N, 4.72; elemental analysis (found, %): C, 28.28; H, 1.1; F, 19.27; N, 4.67.
1,1,3,3-tetrachloro-2-cyano-1,3,-difluoropropan-2-yl acrylate CH2=CH-COO-C(CFCl2)2-CN. To a 500ml three-neck flask 24g of sodium cyanide are added to 250ml of dry acetonitrile while stirring, then the reaction mixture is cooled to 0°C and then 104 g of 1,1,1,3-tetrachloro-1,3-difluoropropan-2-one are added drop by drop. It is being mixed for 30 minutes more at 0°C and for 1,5h at 20°C. Afterwards the reaction mixture is cooled to 0°C and 54,5g of acrylic cid chloroanhydride are added drop by drop. Then the reaction mixture is poured into water. The lower layer is separated, dried with magnesium sulphate, filtered and distilled in vacuum. 113 g of 1,1,3,3-tetrachloro-2-cyano-1,3,-difluoropropan-2-yl acrylate are obtained in the form of colorless liquid. The yield is 80%. The purity is 97%. Boiling point 116-120°C / 20 mm Hg Elemental analysis (calculated %): C, 26.87; H, 0.97; F, 12.14; N, 4.48; elemental analysis (found, %): C, 26.75; H, 1.07; F, 12.1; N, 4.56.
Conclusion
New halogenated monomers based on cyanoperfluoro- and cyanochloroperfluoroacrylates have been synthesized. Monomers’ coefficient of absorption is low (0.04 – 0.51 dB/cm) and their spectrum of refractive indices is wide (from 1.336 to 1.457) in “data communixcation” field of wave length around 0.85 μm. Monomers are capable of radical photopolymerization and can be used for creating different integrated optical devices using methods of UV photolithography and direct laser drawing.
Acknowlegement
The present work has been executed due to financial support of grant of Russian Foundation for Basic Research (RFBR) # 13-03-12265 (in the part of synthesis of halogenated monomers) and of grant # 13-07-00976 (in the part of studying of optical properties of monomers around 0.85 μm).
References
- L.S. Boguslavskaya, A.V.Samarina, V.I. Lebedeva et all, Plast. Massy, 1988, 12, p.15
- Z.S.Salahetdinova, V.N. Golovnenko, G.V. Tsypkina, A.P.Sineokov Osnovnoy organicheskiy sintez i neftehimiya, 1978. Vol. 9. p. 57.
- Akira O., Nobuyuki T., Sakahiro K. EP0128516 (1984).
- Akira O., Nobuyuki T., Takahiro K..EP0128517. (1984)
- Akira O., Takahiro T., Takahiro K. Appl.Japan 60-118808 RJ Chem 1987, 4161. 1574
- Bosc O., Boutevin B., Pietrasanta Y., Rousseau A., FR2623510(1989)
- Jjaa S., Koji N., Maseru M., Takashi I. Appl.Japan 61-121005 C. A. 1986. V. 105, 192481.
- Takashi I., Katsuhiko S., Ryuji M. et al. Appl.Japan 61 240205 C. A. 1987. V. 106, 139496.
- Tategami Y., Fujiia K., Furuta M., Tamura T. Appl.Japan 60-250309 C. A. 1986. V. 104, 226030 p.
- Joshiharu T., Katsuramaru T., Motonobu F., Tashibubnu T. Appl.Japan 61-208006 C. A. 1987. V. 106, 68407.
- Shigeru M., Masahiko O. Appl.Japan 61-86448 C. A. 1986. V. 105, 157727.
- W. Groh, “Overtone absorption in macromolecules for polymer optical fibers”, Macromol. Chem., Vol. 189, pp. 2861-2874, 1998.
- Zhou M. Optical Engineering, 41, 1631 (2002).
- Eldada L. Optical Engineering, 40, 1165 (2001).
- Erdogan T. J. of Lightwave Technology, 15, 1277 (1997).
- Sokolov V.I., Mishakov G.V., Panchenko V.Ya., Tsvetkov M.Yu. Optical Memory and Neural Networks (Information Optics), 16, 67 (2007).
- Molchanov S.I.,Kitay M.S., Sokolov V.I., Troitskaya E.V.” Optical properties of fluorocontaining acrylic monomersand polymers in the telecommunication area about 0,85 mkm wave-length“ “Collection of papers of XII International conference “Physico-chemical processes of atoms and molecules selection in laser, plazma and nonatechnologies.”Ershovo, Moscow region, 2008, p.209-214
- Sokolov V.I., Kitay M.S., Mishakov G.V., Molchanova S.I., Panchenko V.Ya “Spectroscopic refractometer for 375-1150 nm wave-length area PTE, Vol. 1, p. 157-158, 2011
- Igumnov S.M., Sokolov V.I., Men'shikov V.K., Mel'nik O.A., Boyko V.E., Dyachenko V.I., Nikitin L.N., Khaidukov E.V., Yurkov G.Yu., Buznik V.M. Fluorinated monomers and polymers with specific properties for integrated optics and photonics. Doklady Chemistry. 2012., Vol. 446, Iss. 1, p. 183-187.
Recommended for publication by Prof. S. Igoumnov
Fluorine Notes, 2014, 97, 5-6
 a)
a)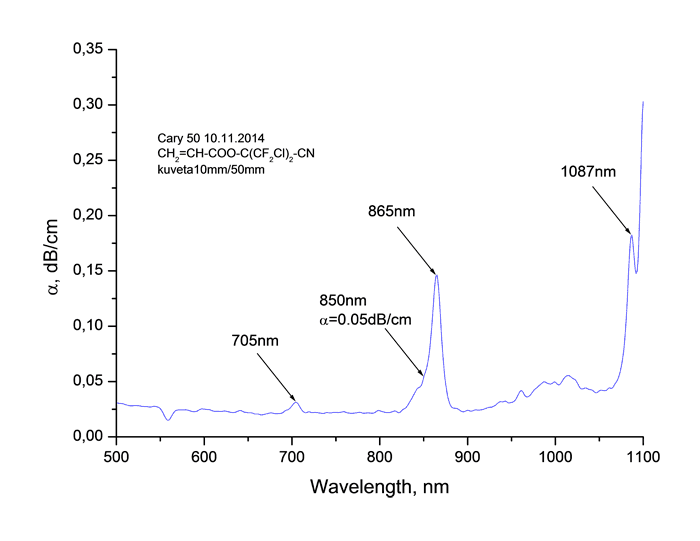 b)
b)